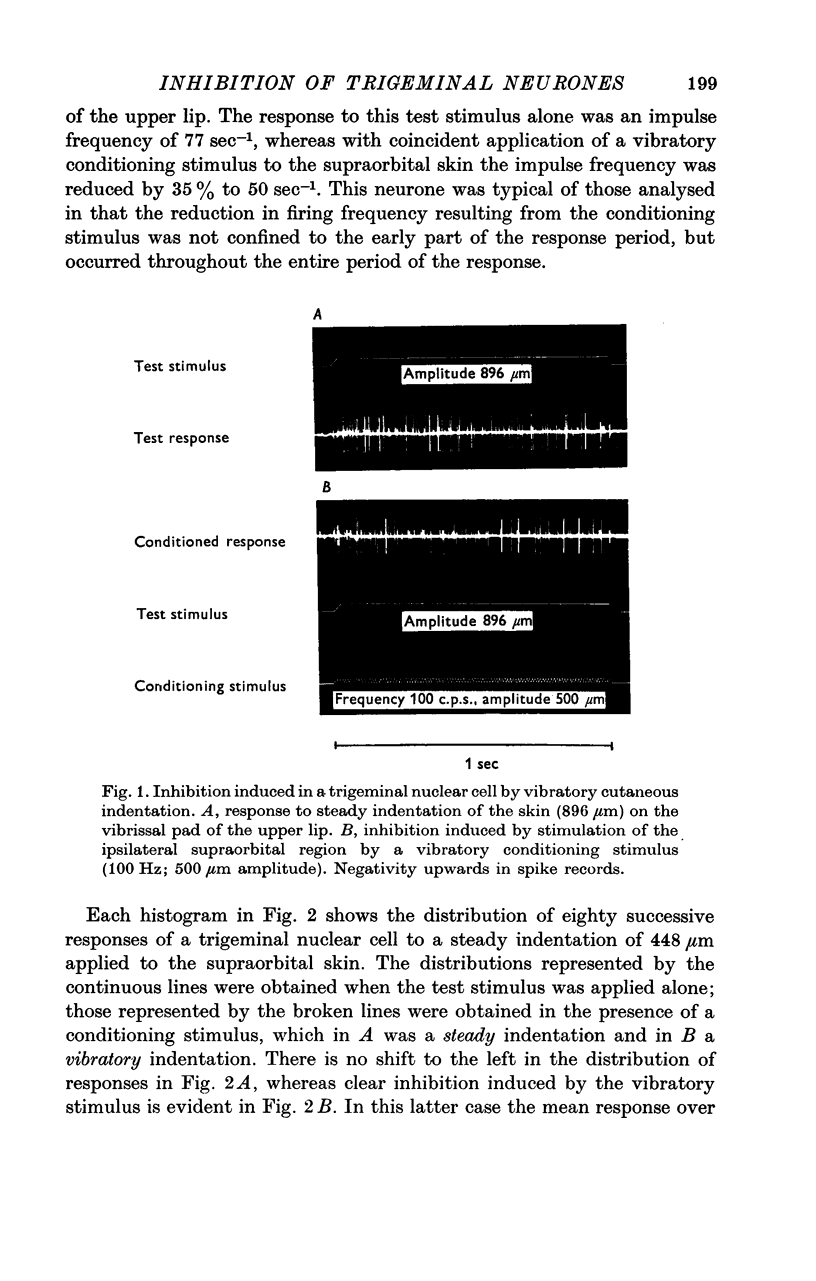
Abstract
1. In decerebrate, unanaesthetized cats two thirds of slowly adapting mechanosensitive neurones sampled in the trigeminal nucleus oralis exhibited inhibition in response to conditioning mechanical stimulation applied beyond their excitatory receptive fields. The influence of this inhibition was examined over the response range of these neurones using controlled, reproducible natural stimulation procedures.
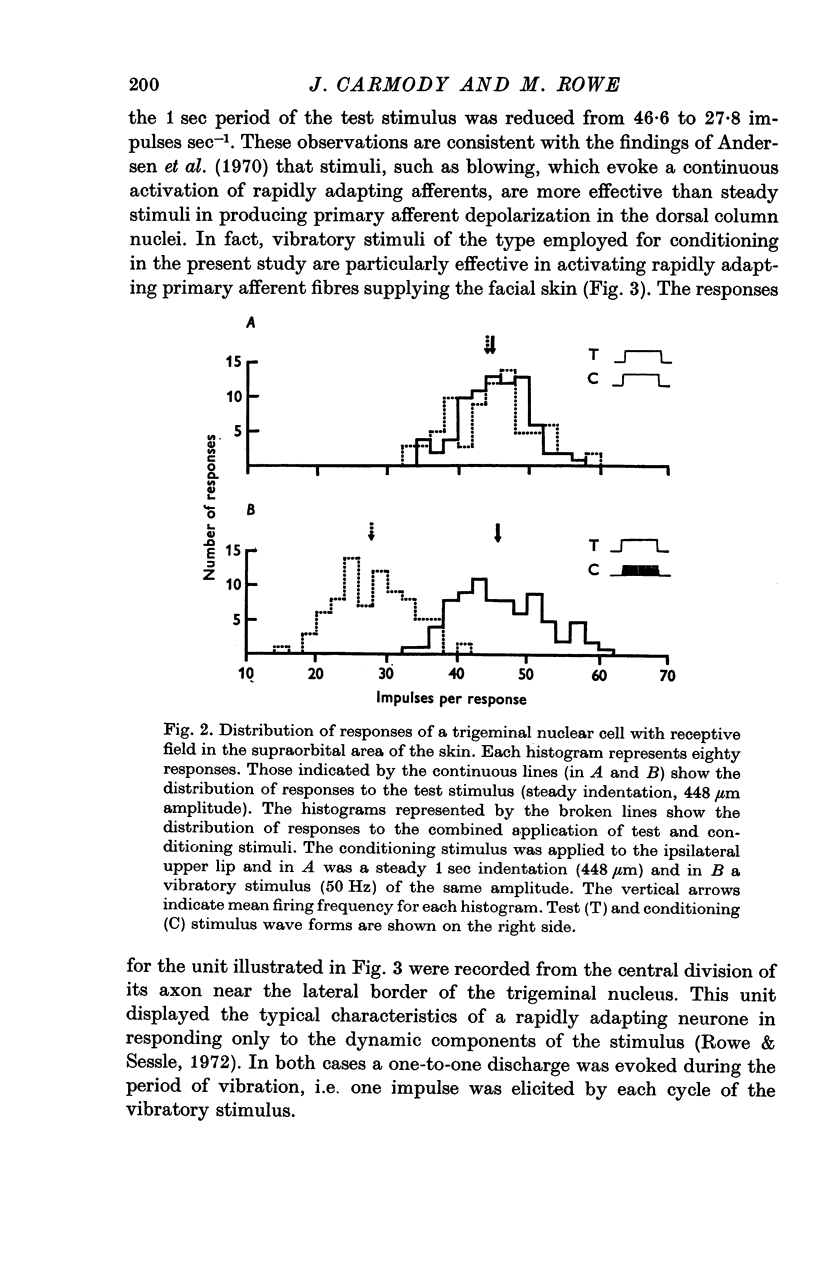
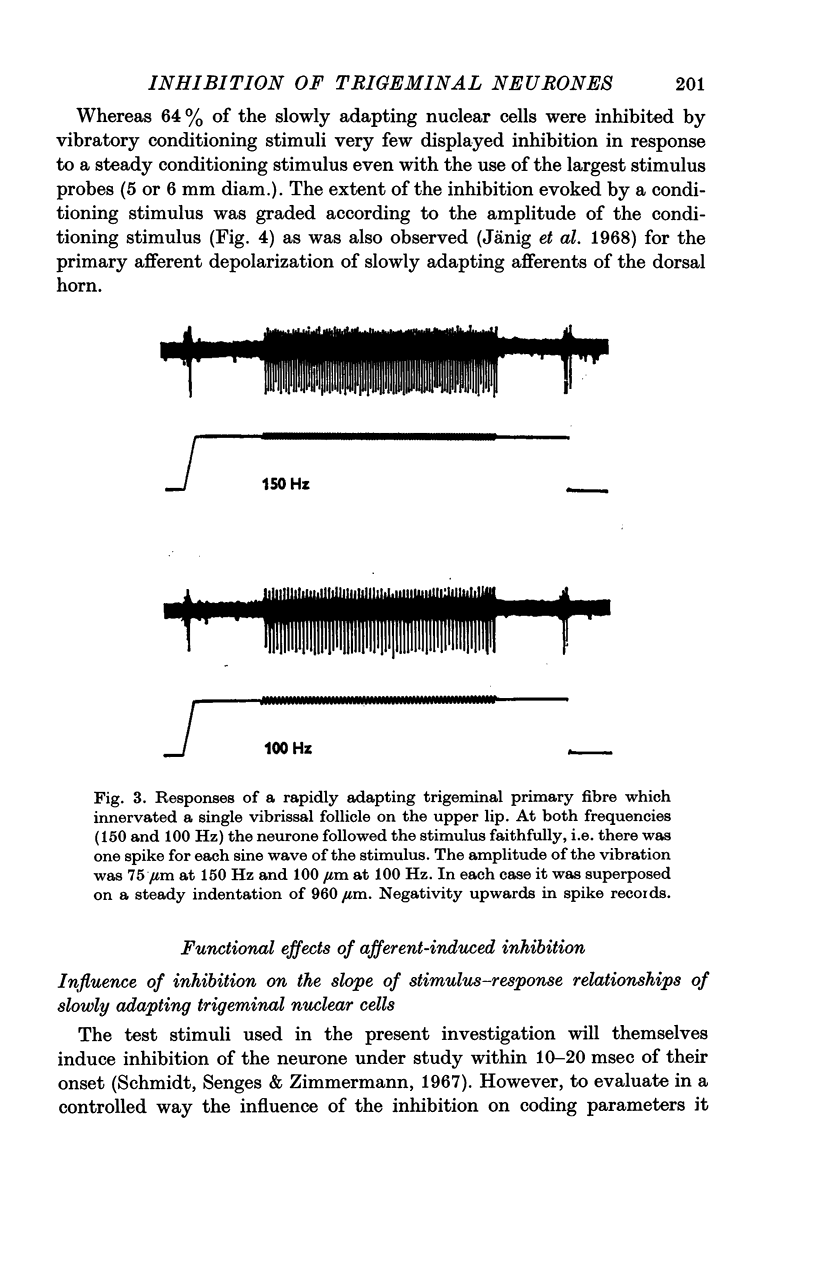
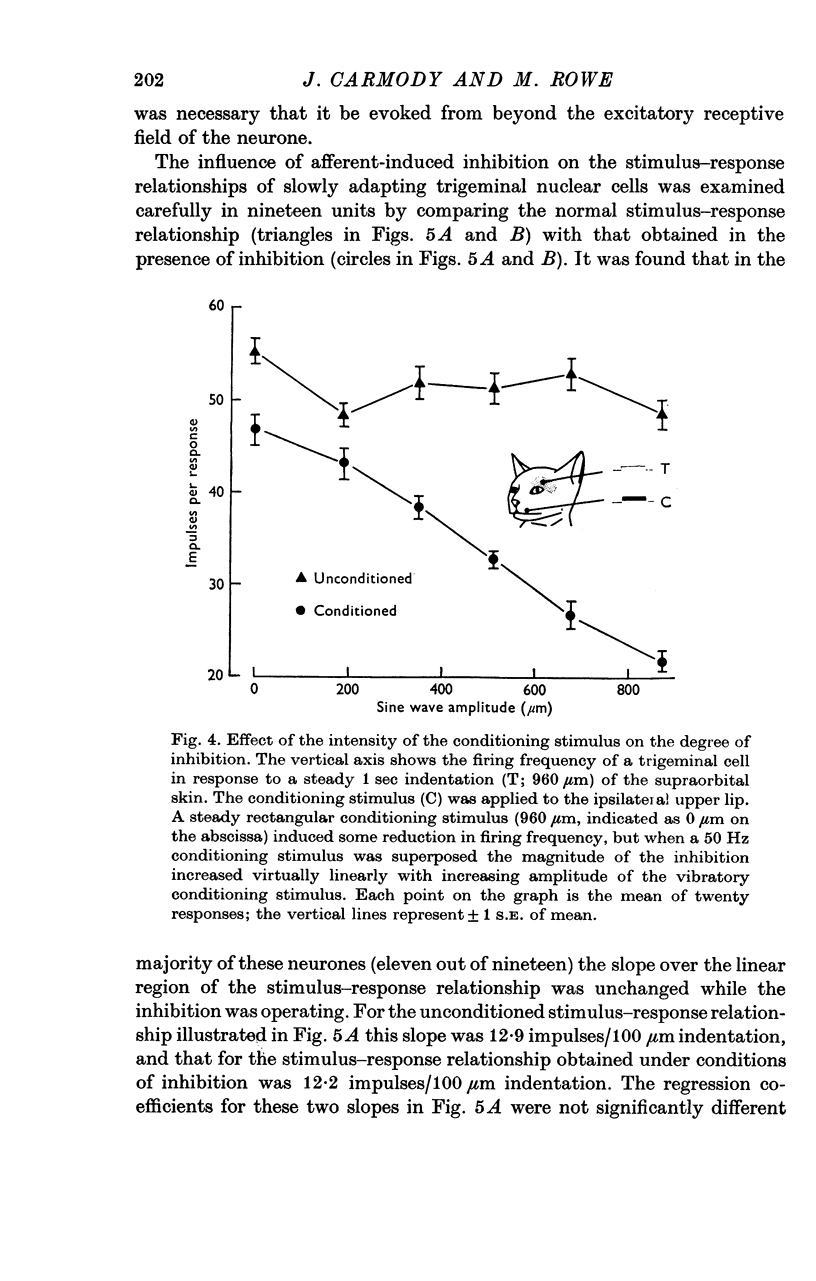
2. The extent of the inhibition was graded according to the intensity of the conditioning stimulus. It was evoked most strongly by vibratory skin indentation which very effectively excites rapidly adapting afferent fibres. Tonic conditioning inputs associated with steady skin indentation were less effective.
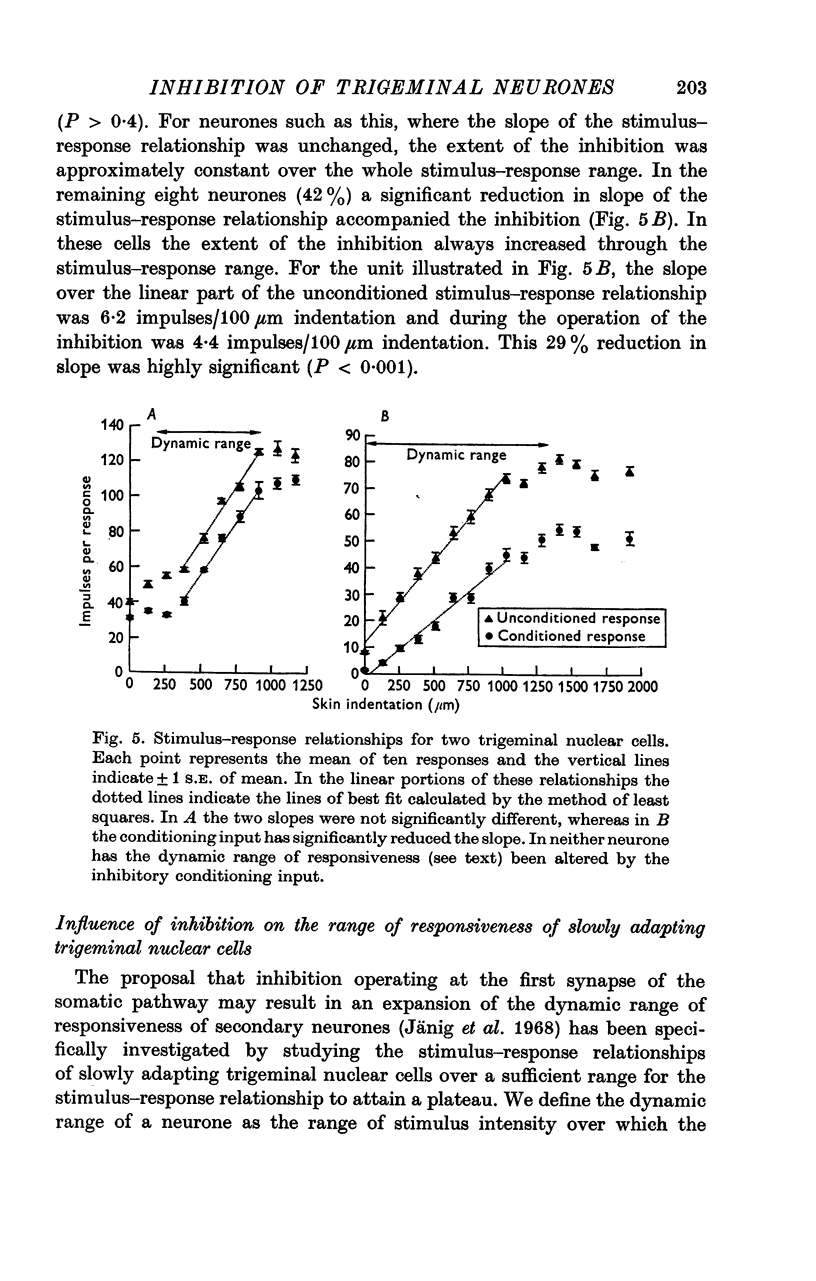
3. The slope of stimulus—response relationships constructed from responses to inputs from the excitatory receptive field was reduced in 42% of trigeminal nuclear cells in the presence of afferent-induced inhibition. In the remainder the slope was unchanged.
4. There was no evidence, in the neurones subject to inhibition, of an expansion of their dynamic range defined as the range of stimulus intensities over which a neurone exhibited a graded responsiveness.
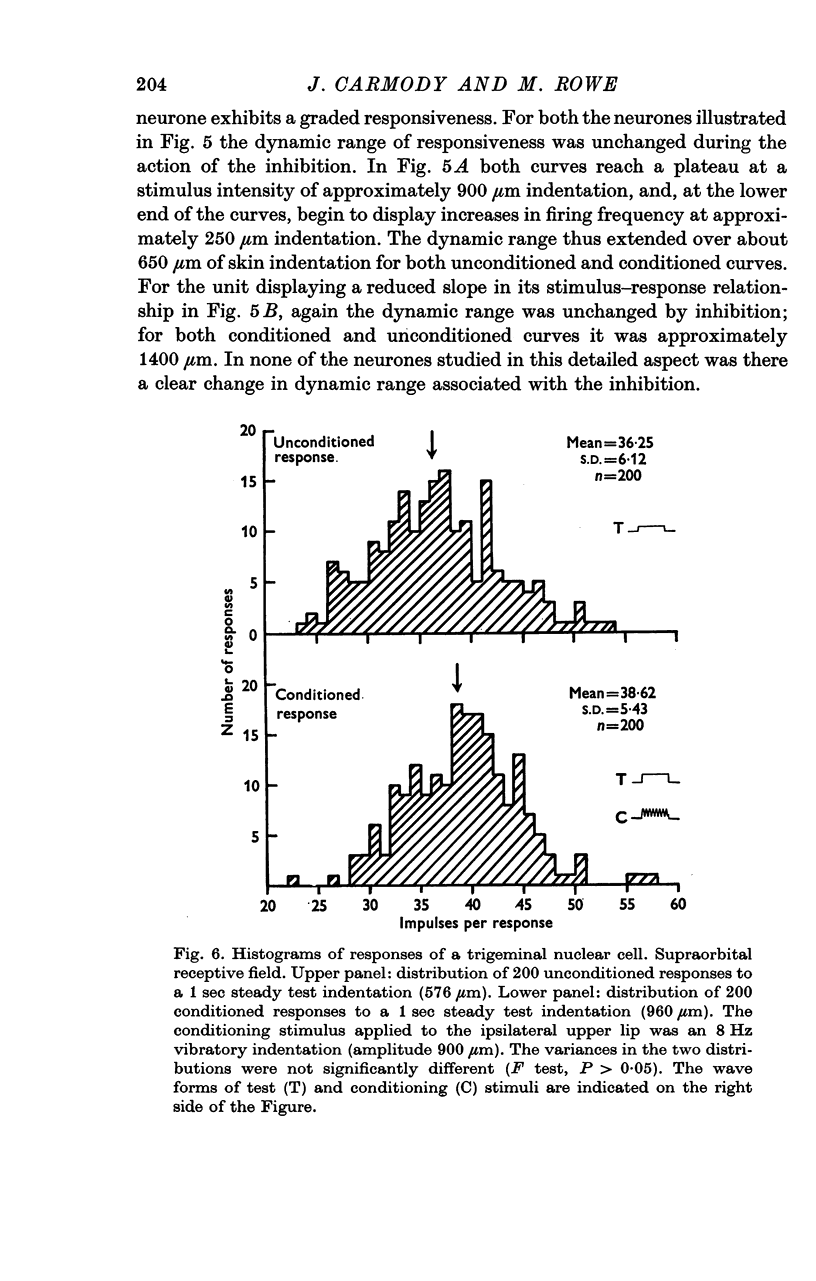
5. The variability in responses of an individual neurone at a given stimulus intensity was unchanged by this inhibition.
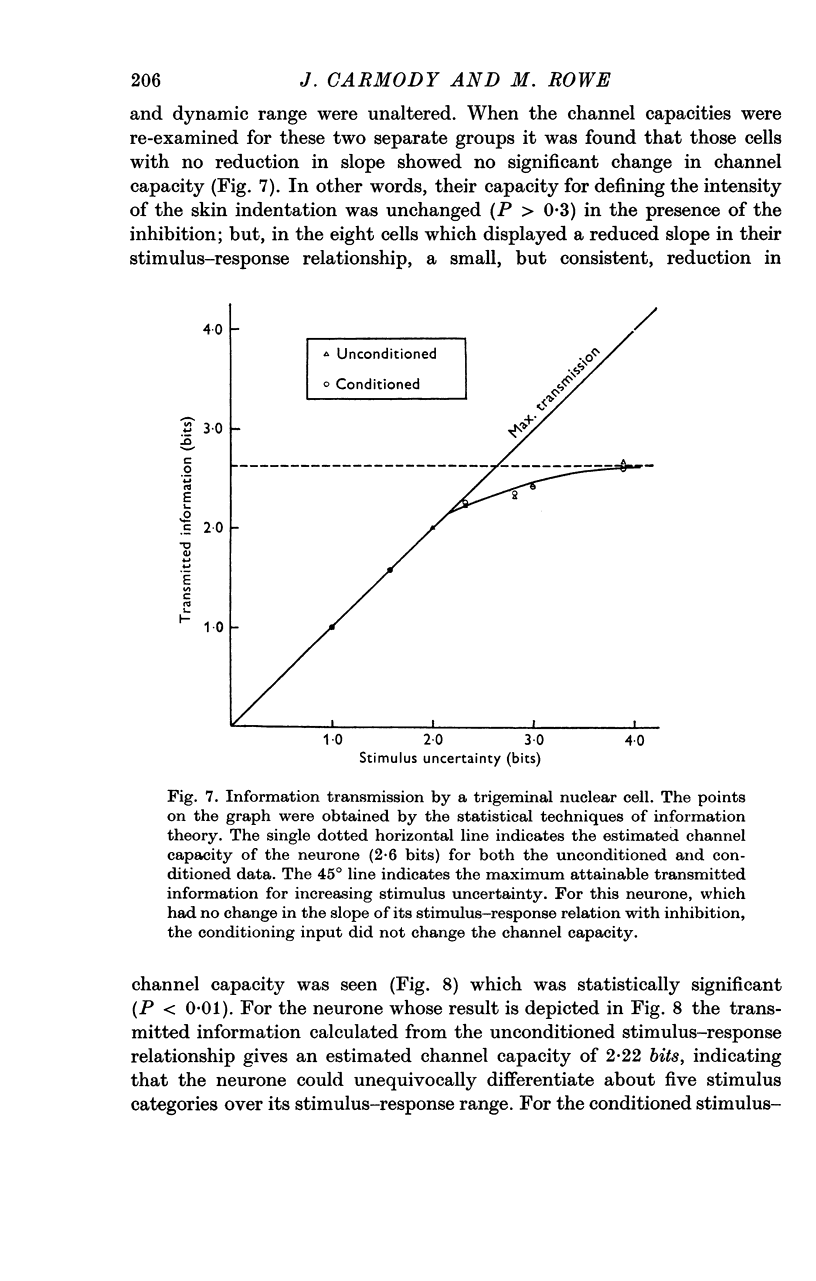
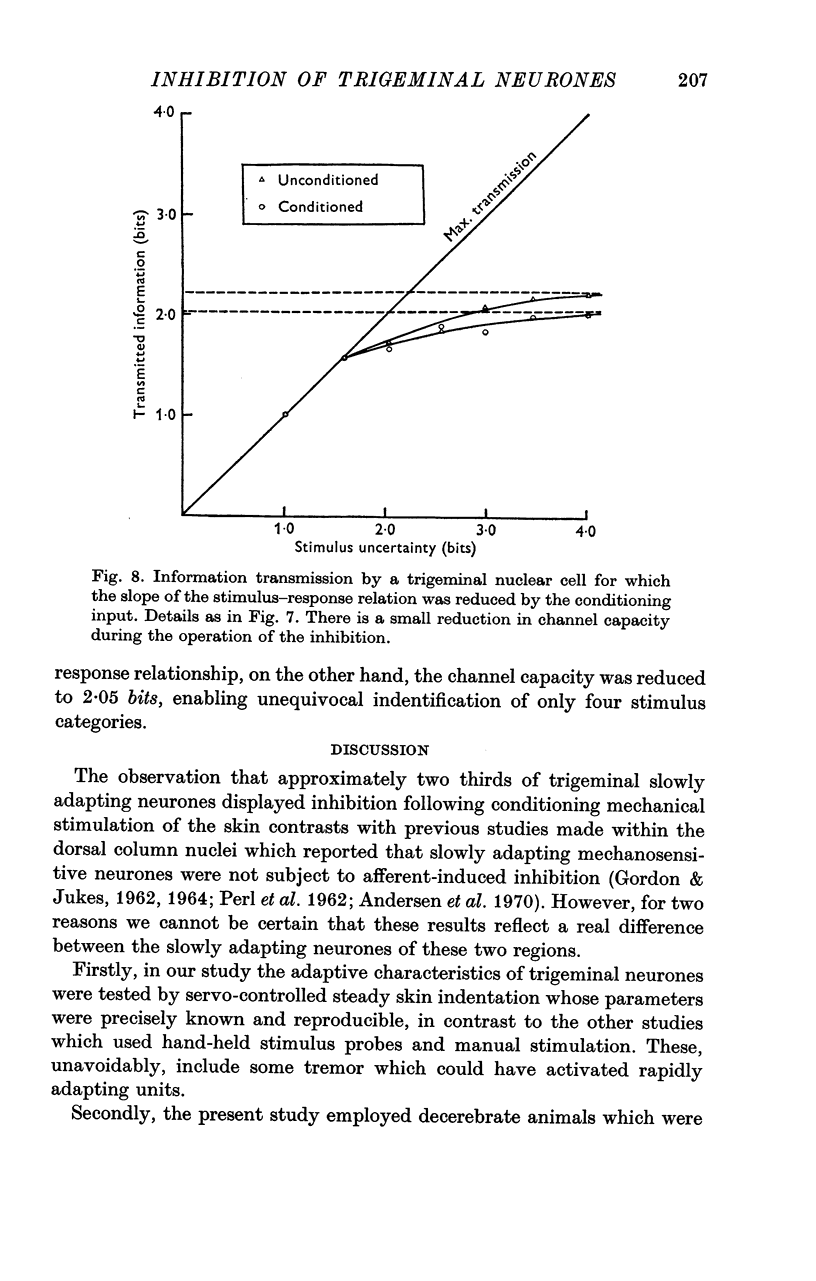
6. Analysis of the stimulus—response data using information theory statistics revealed that neurones which underwent a reduction in the slope of their stimulus—response relationship in the presence of inhibition displayed a reduced capacity for defining the intensity of skin indentation. This capacity was not modified in those neurones where the slope was unchanged by the peripherally evoked inhibition.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I. PRESYNAPTIC COMPONENT IN THE AFFERENT INHIBITION OBSERVED WITHIN TRIGEMINAL BRAIN-STEM NUCLEI OF THE CAT. J Neurophysiol. 1965 Jul;28:695–709. doi: 10.1152/jn.1965.28.4.695. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I., Yokota T. Corticofugal effects on different neuron types within the cat's brain stem activated by tactile stimulation of the face. J Neurophysiol. 1966 Mar;29(2):185–206. doi: 10.1152/jn.1966.29.2.185. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. Correlation of different excitatory and inhibitory influences on cells in the nucleus gracilis of the cat. Nature. 1962 Dec 22;196:1183–1185. doi: 10.1038/1961183a0. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., LANDGREN S., SEED W. A. The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol. 1961 Oct;158:544–559. doi: 10.1113/jphysiol.1961.sp006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PAINE C. H. Functional organization in nucleus gracilis of the cat. J Physiol. 1960 Sep;153:331–349. doi: 10.1113/jphysiol.1960.sp006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- MILLER G. A. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956 Mar;63(2):81–97. [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- OLSZEWSKI J. On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol. 1950 Jun;92(3):401–413. doi: 10.1002/cne.900920305. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Carmody J. J. Afferent inhibition over the response range of secondary trigeminal neurones. Brain Res. 1970 Mar 3;18(2):371–374. doi: 10.1016/0006-8993(70)90338-0. [DOI] [PubMed] [Google Scholar]
- Rowe M. J. Reduction of response variability in the somatic sensory system by conditioning inputs. Brain Res. 1970 Sep 16;22(3):417–420. doi: 10.1016/0006-8993(70)90486-5. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Responses of trigeminal ganglion and brain stem neurones in the cat to mechanical and thermal stimulation of the face. Brain Res. 1972 Jul 20;42(2):367–384. doi: 10.1016/0006-8993(72)90537-9. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Somatic afferent input to posterior thalamic neurones and their axon projection to the cerebral cortex in the cat. J Physiol. 1968 May;196(1):19–35. doi: 10.1113/jphysiol.1968.sp008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. F., Senges J., Zimmermann M. Presynaptic depolarization of cutaneous mechanoreceptor afferents after mechanical skin stimulation. Exp Brain Res. 1967;3(3):234–247. doi: 10.1007/BF00235587. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Trautwein W., Zimmermann M. Dorsal root potentials evoked by natural stimulation of cutaneous afferents. Nature. 1966 Oct 29;212(5061):522–523. doi: 10.1038/212522a0. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]


