Abstract
1. Single giant barnacle muscle fibres from Megabalanus psittacus (Darwin) were used to measure the Ca entry and the development of tension in the fibres under membrane potential control.
2. Fibres bathing in 60 mm-MgCl2 sea water, free of Ca, did not develop tension with sudden displacements of the membrane potential towards more positive values. This failure to develop tension with depolarizations was observed with and without the internal application of Ca buffers.
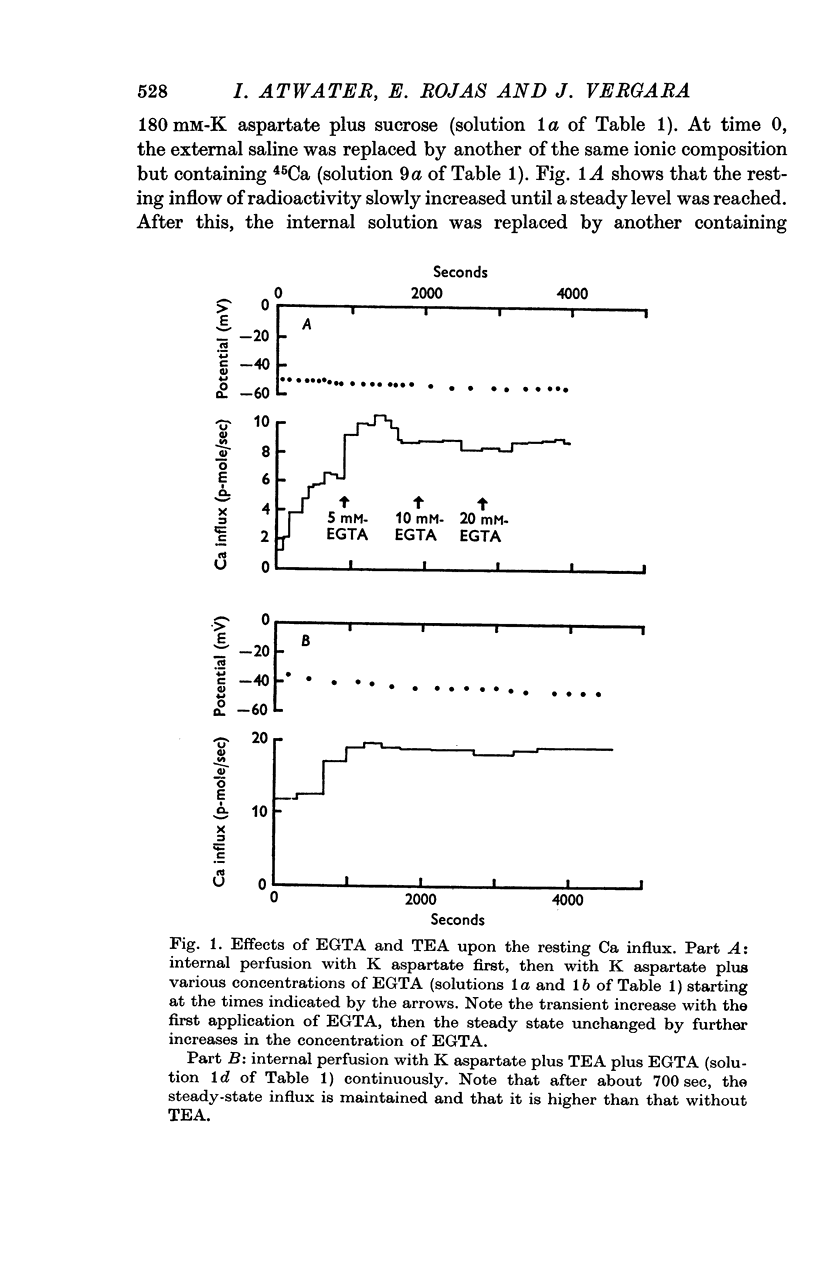
3. Fibres bathing in artificial sea water with either 10, 20, 60 or 100 mm-CaCl2 developed tension with depolarization even after 60 min of internal perfusion of the fibres with solution containing no Ca buffers. In this case the maximum tension recorded during a voltage clamp run decreased with time from nearly 2·5 to 0·2 kg/cm2. However, addition of 10 mm-Tris-EGTA (ethyleneglycol-bis (β-aminoethyl ether) N, N′ — tetraacetic acid) to the perfusing solution rapidly eliminated the development of tension; after 10 min of internal perfusion with Ca buffers no tension could be elicited by electrical stimulation.
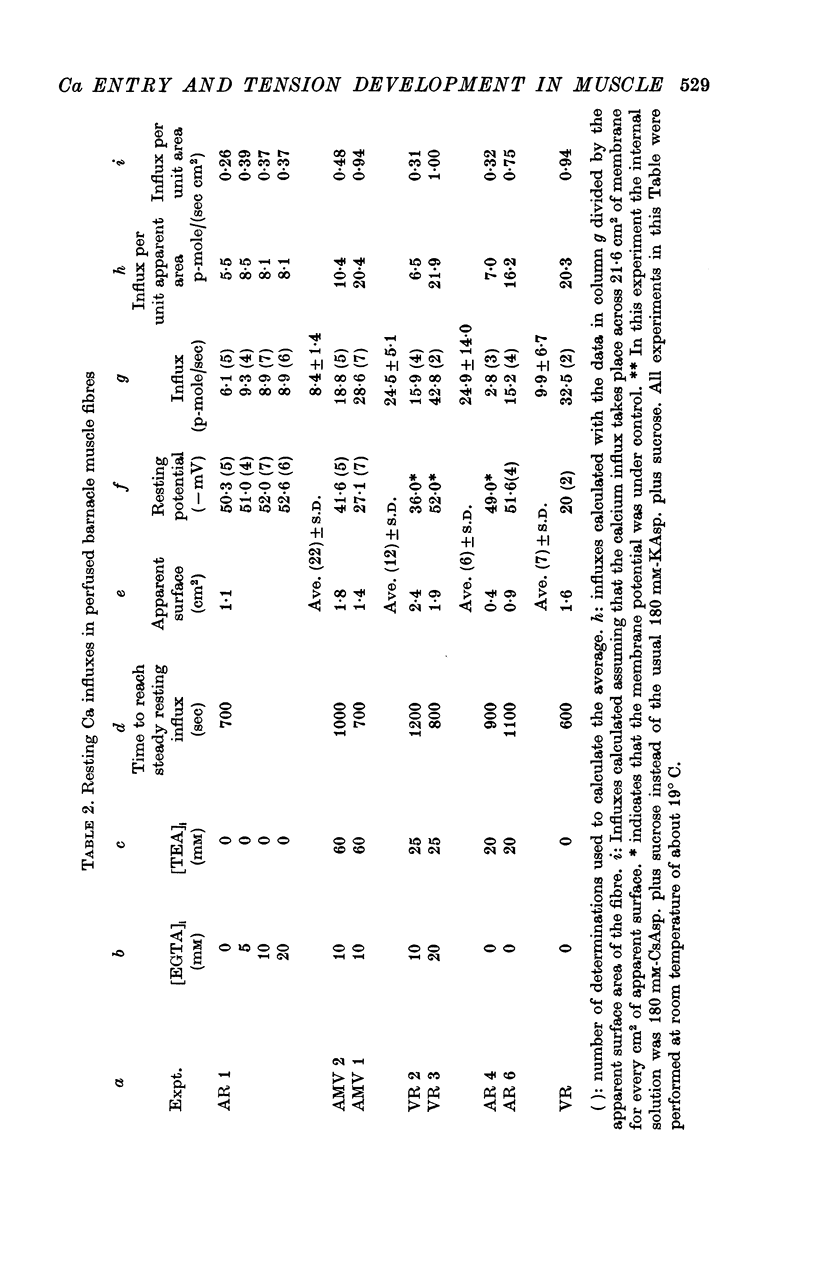
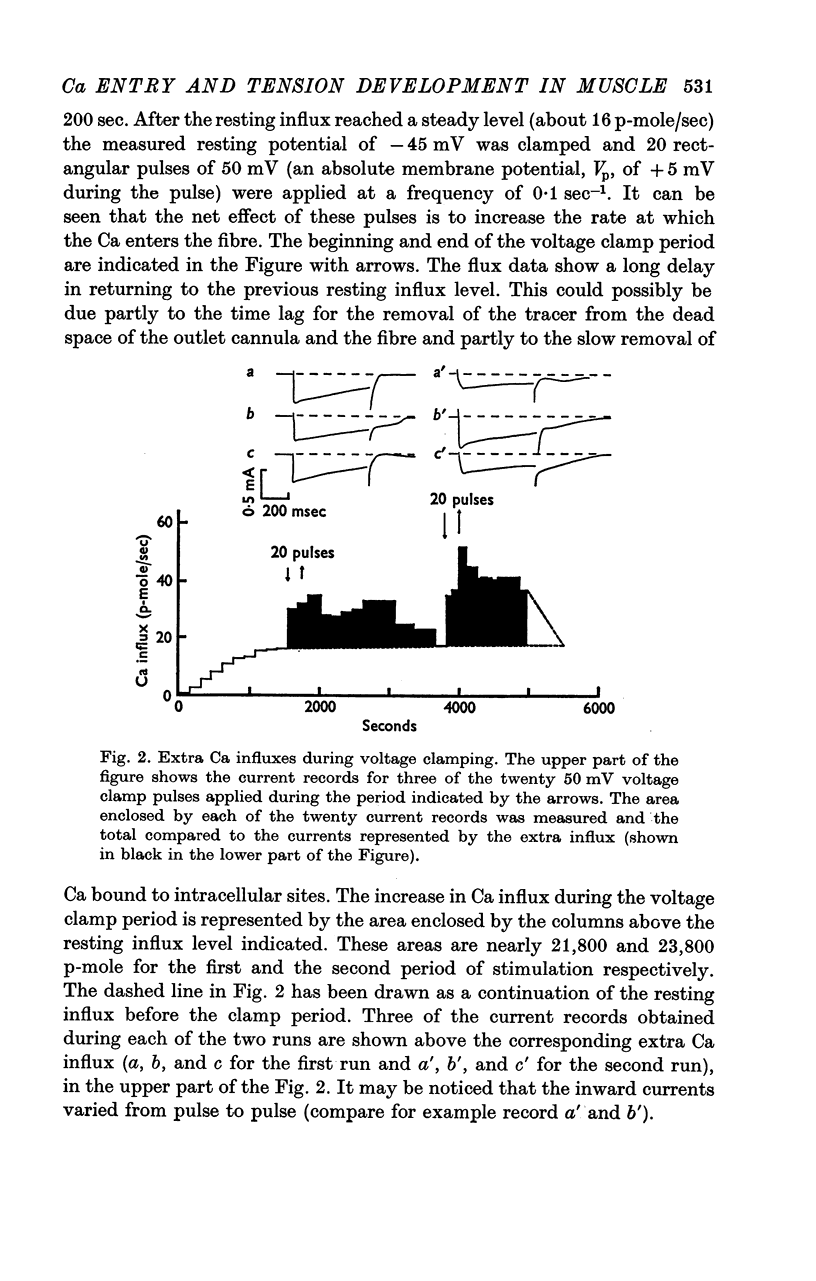
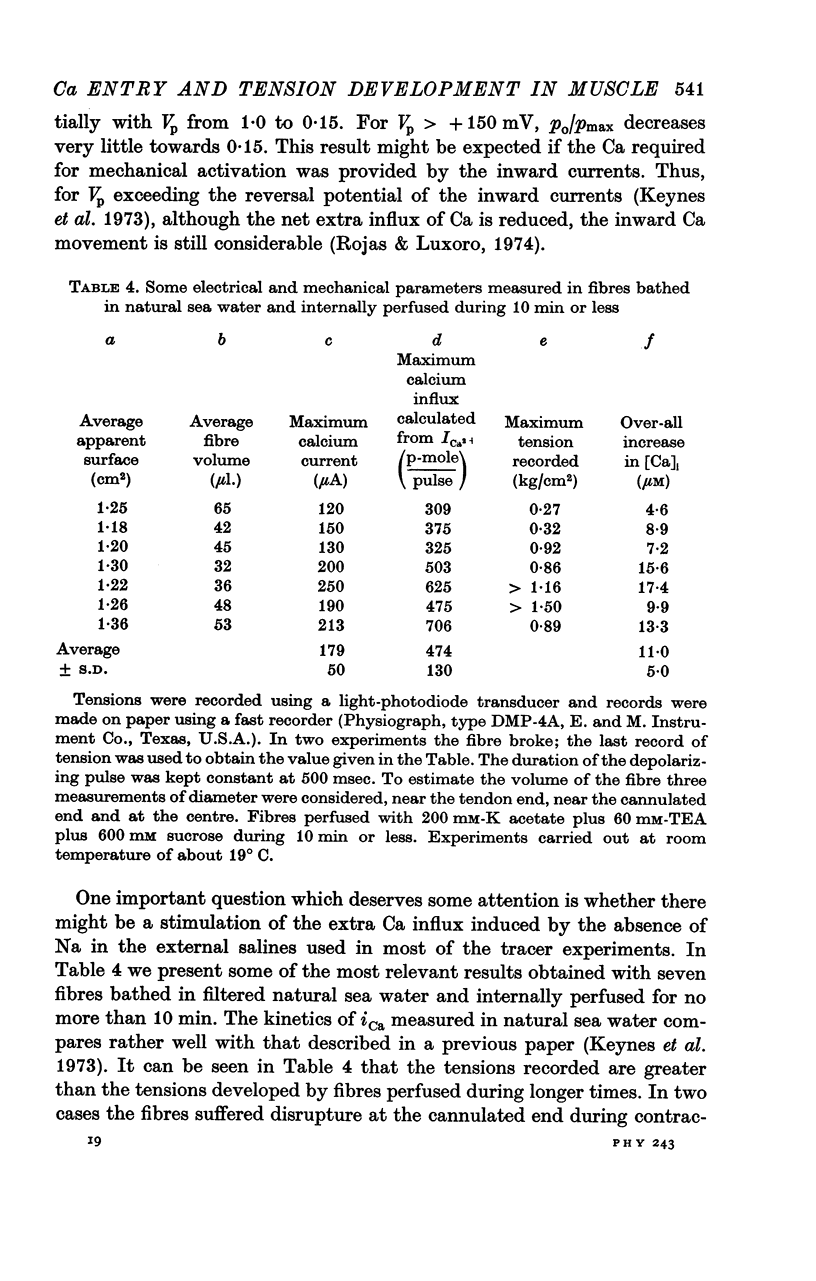
4. Ca-influx determinations were carried out only in the fibres in which the outward K+ currents were blocked by internal application of TEA (tetra-ethylammonium). The ratio of `measured extra Ca influx/computed ionic flux of divalent cations during the inward current' was 1·06 ± 0·41.
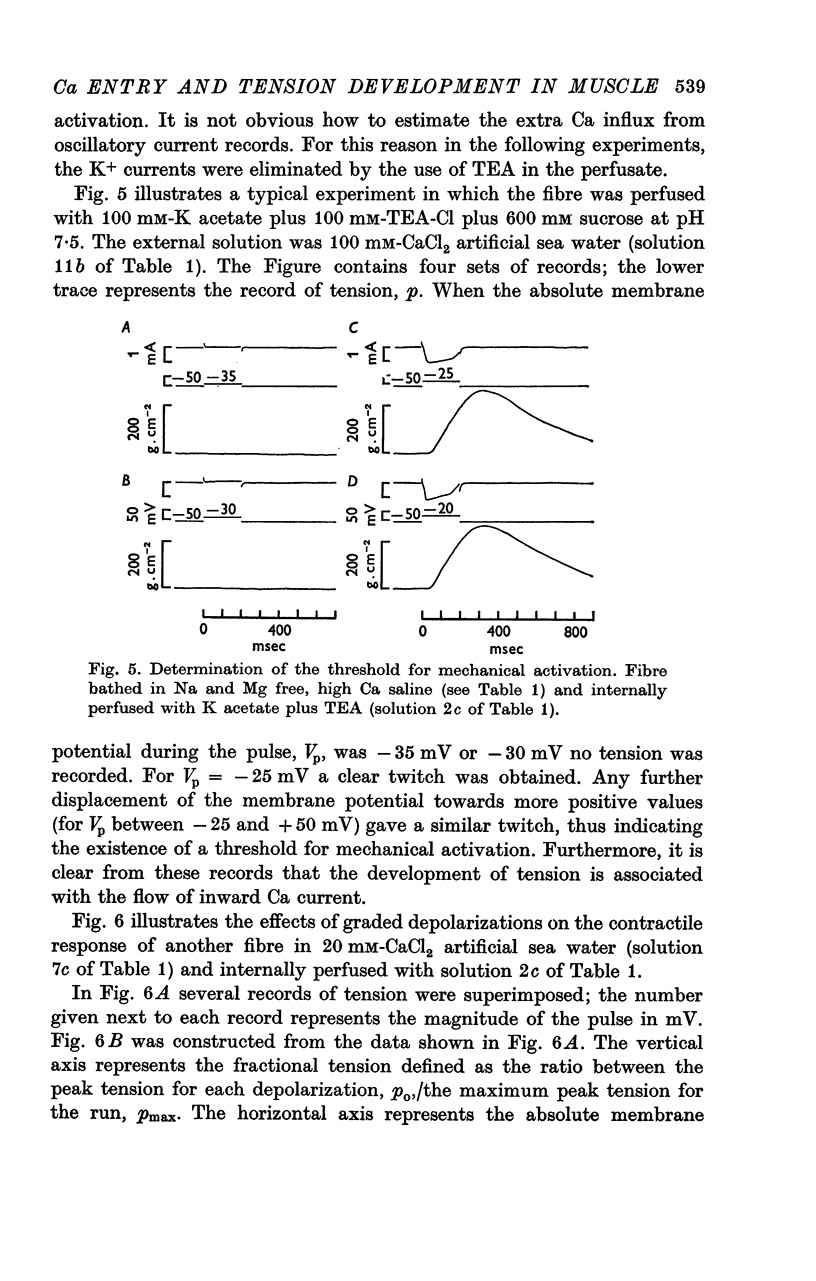
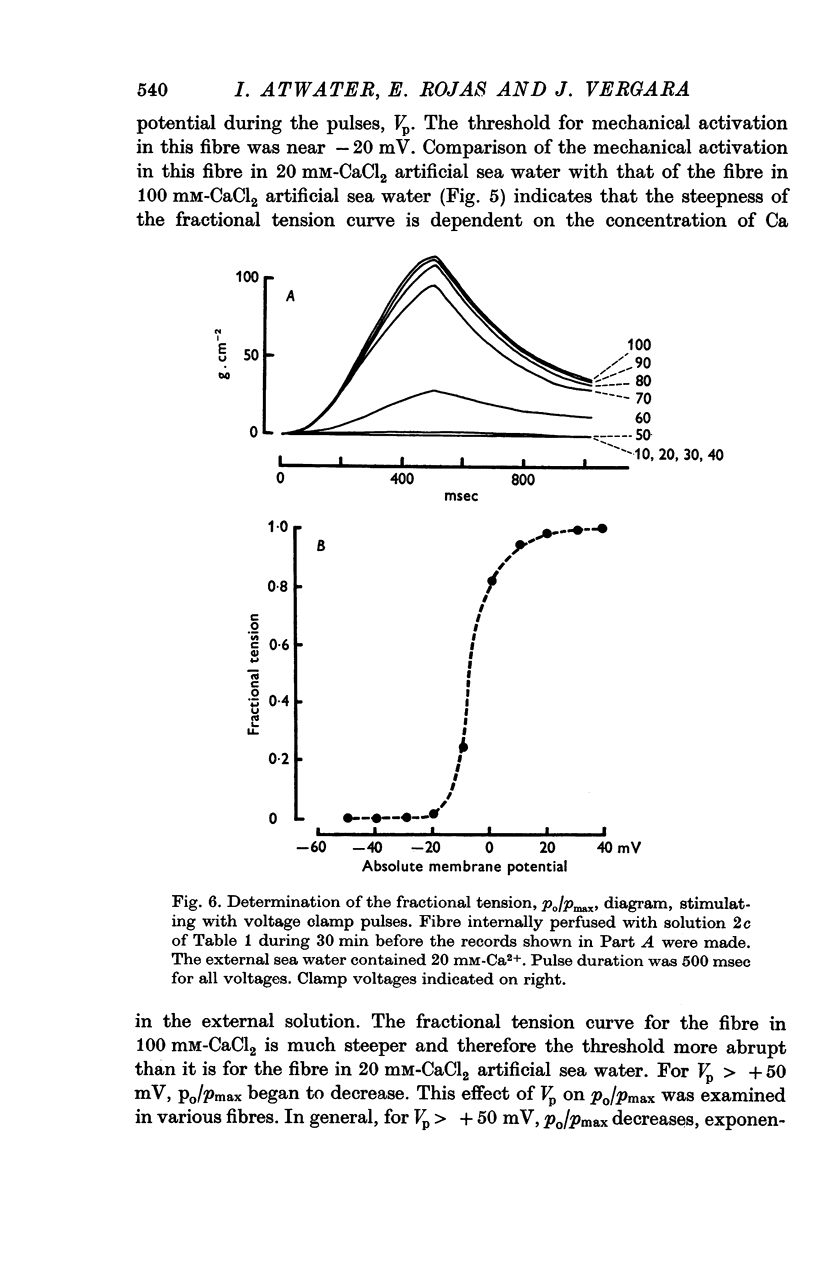
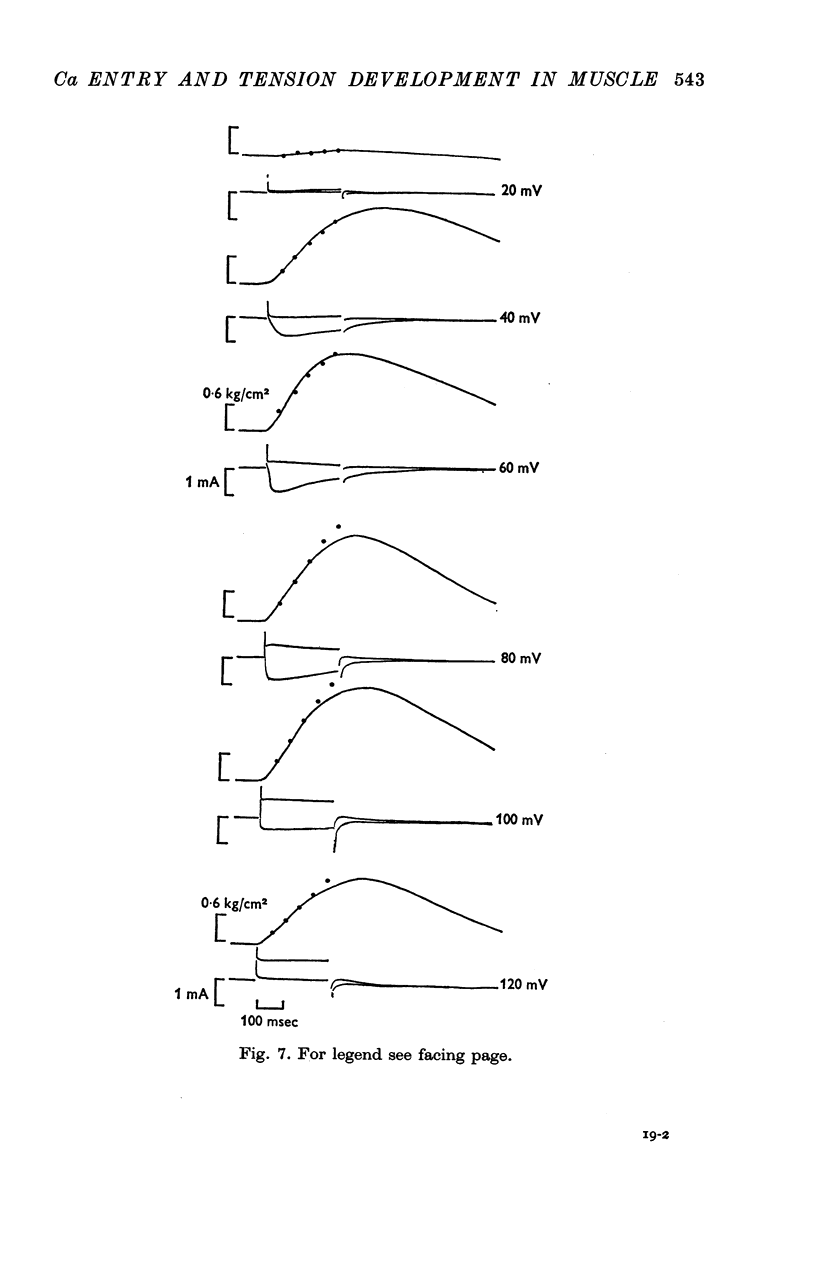
5. For fibres bathed in either natural sea water or in artificial sea water with various concentrations of Ca, the temporal course of development of isometric tension was similar to the temporal course of the integral of the inward current due to Ca2+.
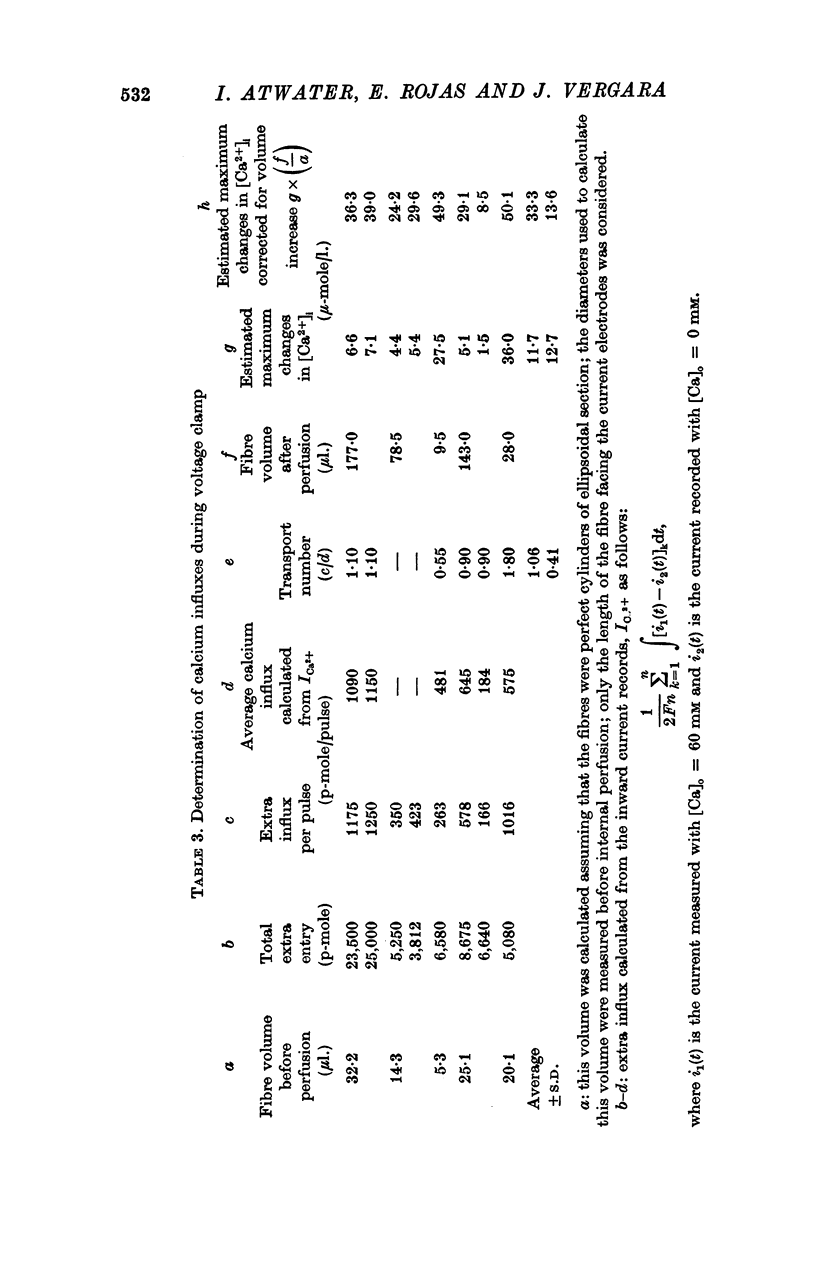
6. In a fibre from M. psittacus bathing in natural sea water the calculated extra entry of Ca required to increase its internal concentration to about 50 μm was 500 p-mole per depolarization (60 mV); while the corresponding average influx calculated from the inward current record in natural sea water is 474 p-mole.
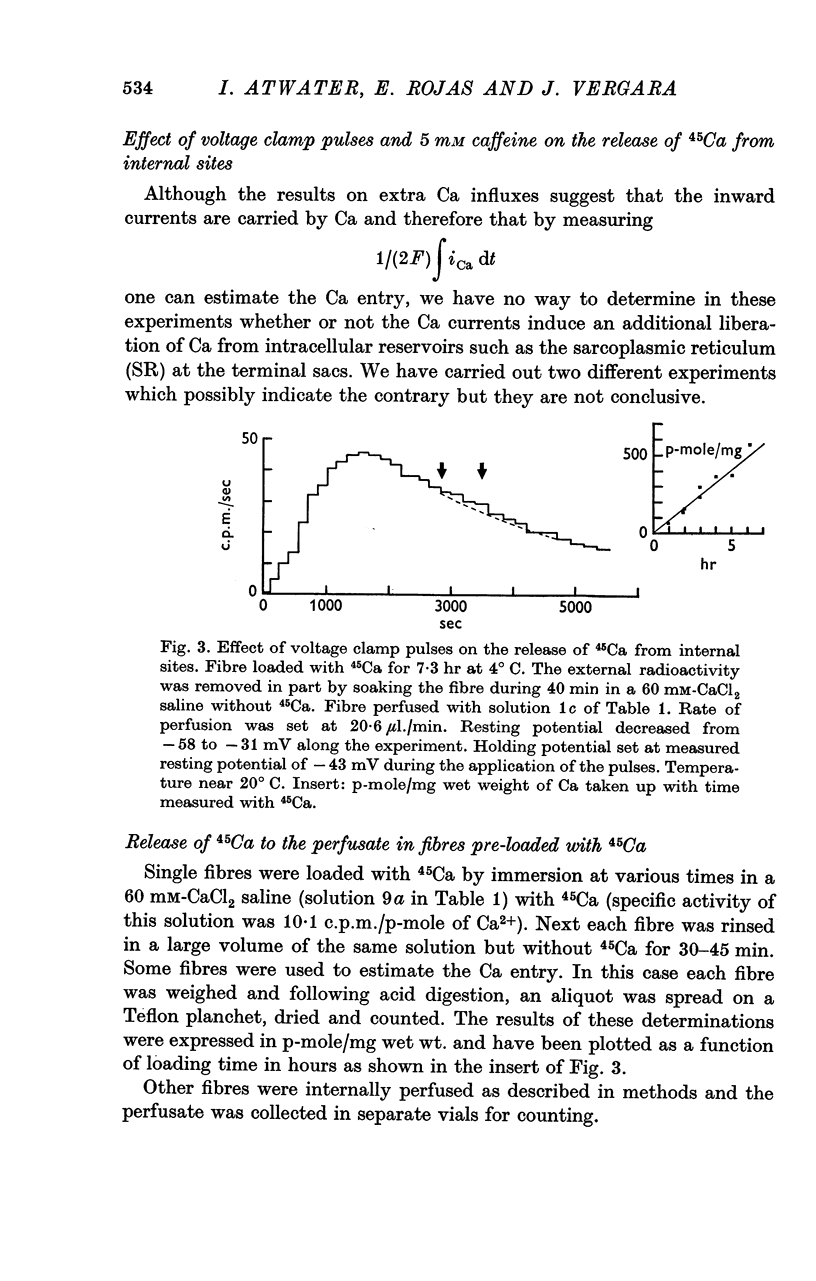
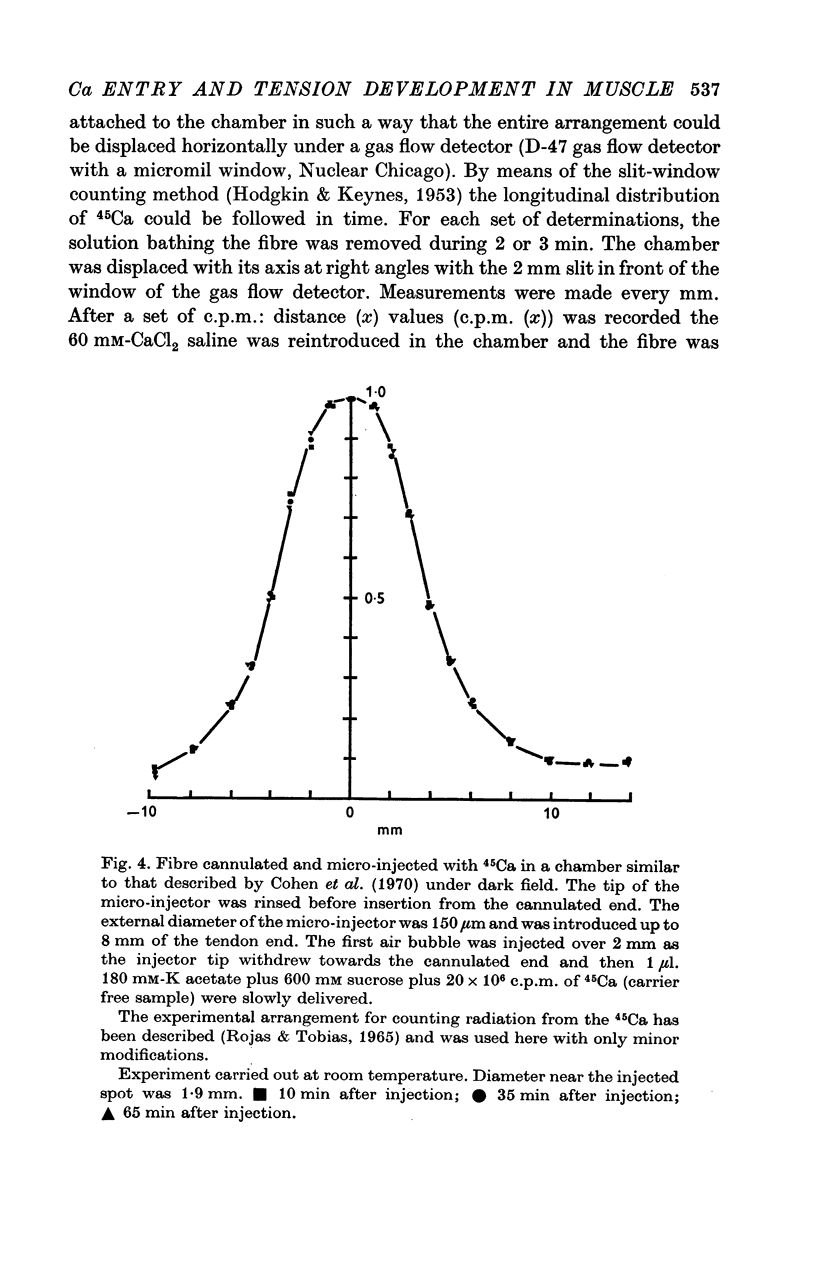
7. Evidence was obtained for the accumulation of Ca in an internal compartment.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Caldwell P. C., Lowe A. G. The efflux of calcium from single crab and barnacle muscle fibres. J Physiol. 1972 Jun;223(3):735–755. doi: 10.1113/jphysiol.1972.sp009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C. The role of cell calcium in the contraction of single cannulated muscle fibers. Am Zool. 1967 Aug;7(3):647–659. doi: 10.1093/icb/7.3.647. [DOI] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin R. J. Ultrastructure and calcium transport in crustacean muscle microsomes. J Cell Biol. 1971 Jan;48(1):49–60. doi: 10.1083/jcb.48.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Caputo C., Gonzalez-Serratos H., Venosa R. A. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972 Jun;223(2):507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Time course of the sodium influx in squid giant axon during a single voltage clamp pulse. J Physiol. 1970 Mar;207(1):151–164. doi: 10.1113/jphysiol.1970.sp009054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Changes in axon birefringence during the action potential. J Physiol. 1970 Dec;211(2):495–515. doi: 10.1113/jphysiol.1970.sp009289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- Dreizen P., Gershman L. C., Trotta P. P., Stracher A. Subunits and their interactions. J Gen Physiol. 1967 Jul;50(6 Suppl):85–118. doi: 10.1085/jgp.50.6.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The mobility and diffusion coefficient of potassium in giant axons from Sepia. J Physiol. 1953 Mar;119(4):513–528. doi: 10.1113/jphysiol.1953.sp004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K., Junge D. Excitation-contraction coupling in a barnacle muscle fiber as examined with voltage clamp technique. J Gen Physiol. 1968 Feb;51(2):157–175. doi: 10.1085/jgp.51.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G., McNeill P. A., Selverston A. I. Ultrastructure of barnacle giant muscle fibers. J Cell Biol. 1973 Jan;56(1):74–91. doi: 10.1083/jcb.56.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanaiah N., Rojas E. Effects of anions and cations on the resting membrane potential of internally perfused barnacle muscle fibres. J Physiol. 1973 Sep;233(3):613–634. doi: 10.1113/jphysiol.1973.sp010326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. Movements of Ca in beating ventricles of the frog heart. J Physiol. 1963 Jul;167:551–580. doi: 10.1113/jphysiol.1963.sp007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The potassium chloride contracture of the heart and its modification by calcium. J Physiol. 1956 Dec 28;134(3):584–599. doi: 10.1113/jphysiol.1956.sp005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- ROJAS E., TOBIAS J. M. MEMBRANE MODEL: ASSOCIATION OF INORGANIC CATIONS WITH PHOSPHOLIPID MONOLAYERS. Biochim Biophys Acta. 1965 Mar 29;94:394–404. doi: 10.1016/0926-6585(65)90047-6. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. G., Glovsky J. The uptake of Ca2+ and Sr2+ by fractions from lobster muscle. Comp Biochem Physiol. 1965 Aug;15(4):547–565. doi: 10.1016/0010-406x(65)90154-4. [DOI] [PubMed] [Google Scholar]


