Abstract
1. The effect of La3+ on amylase release and Ca2+ fluxes in mouse pancreatic fragments in vitro was studied.
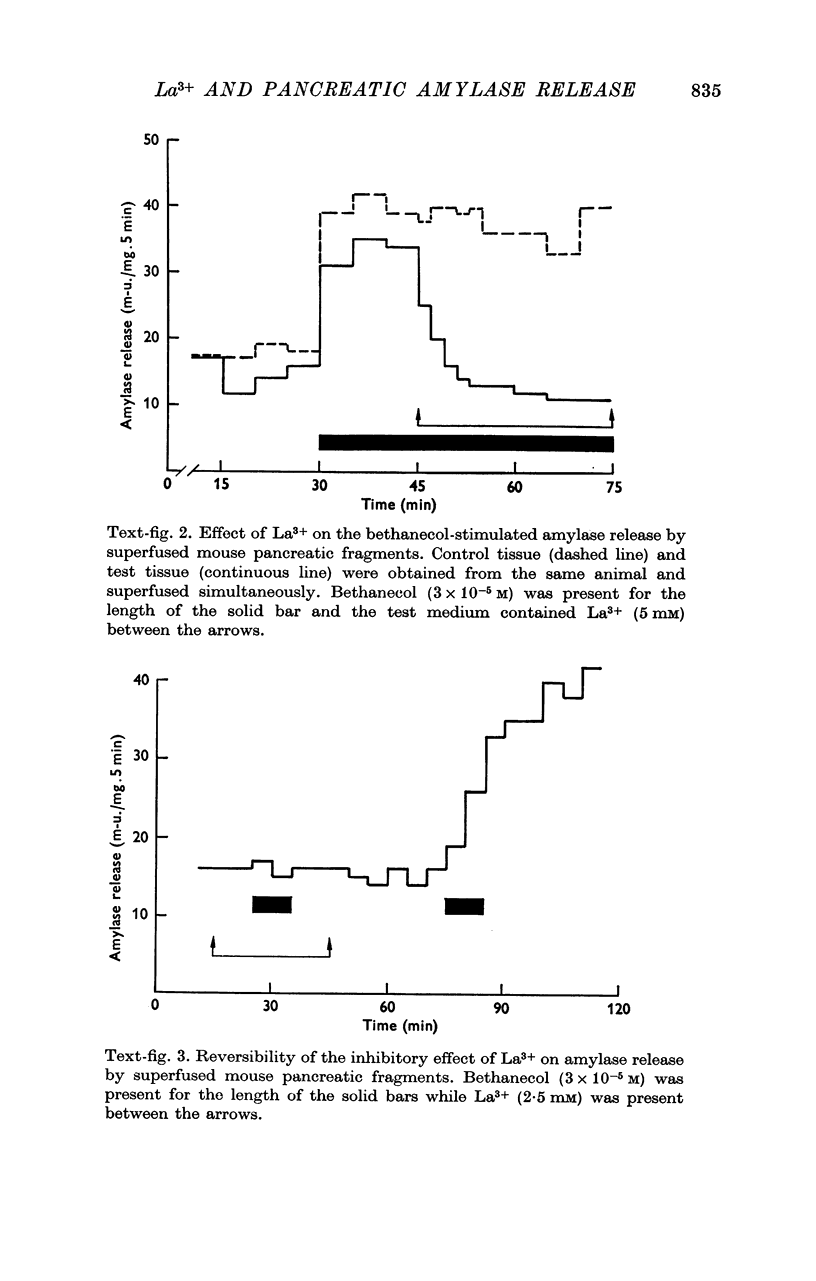
2. Amylase release was increased by 0·1 mM-La3+ and progressively inhibited by 1·0-10 mM-La3+. Non-stimulated and bethanecol stimulated secretion were altered in an identical manner. Inhibition of amylase release was rapid and reversible.
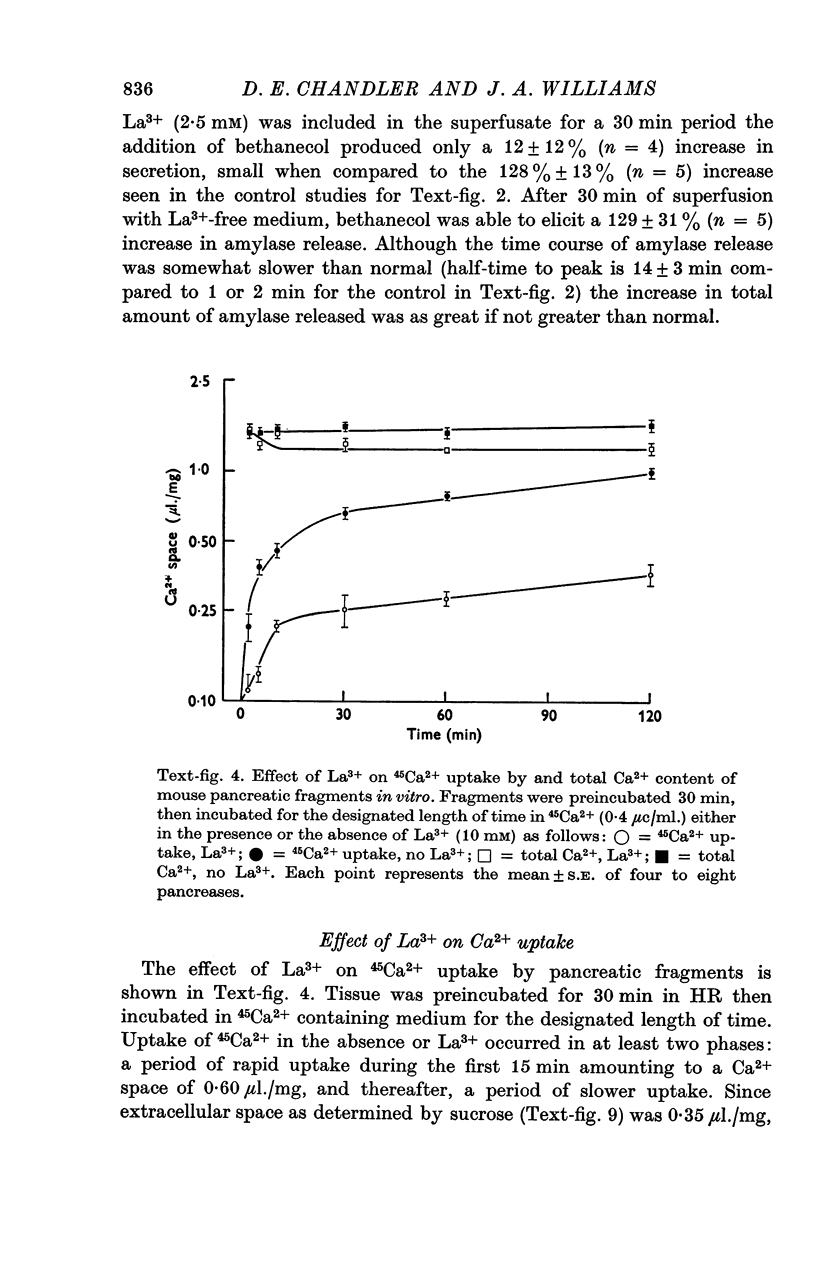
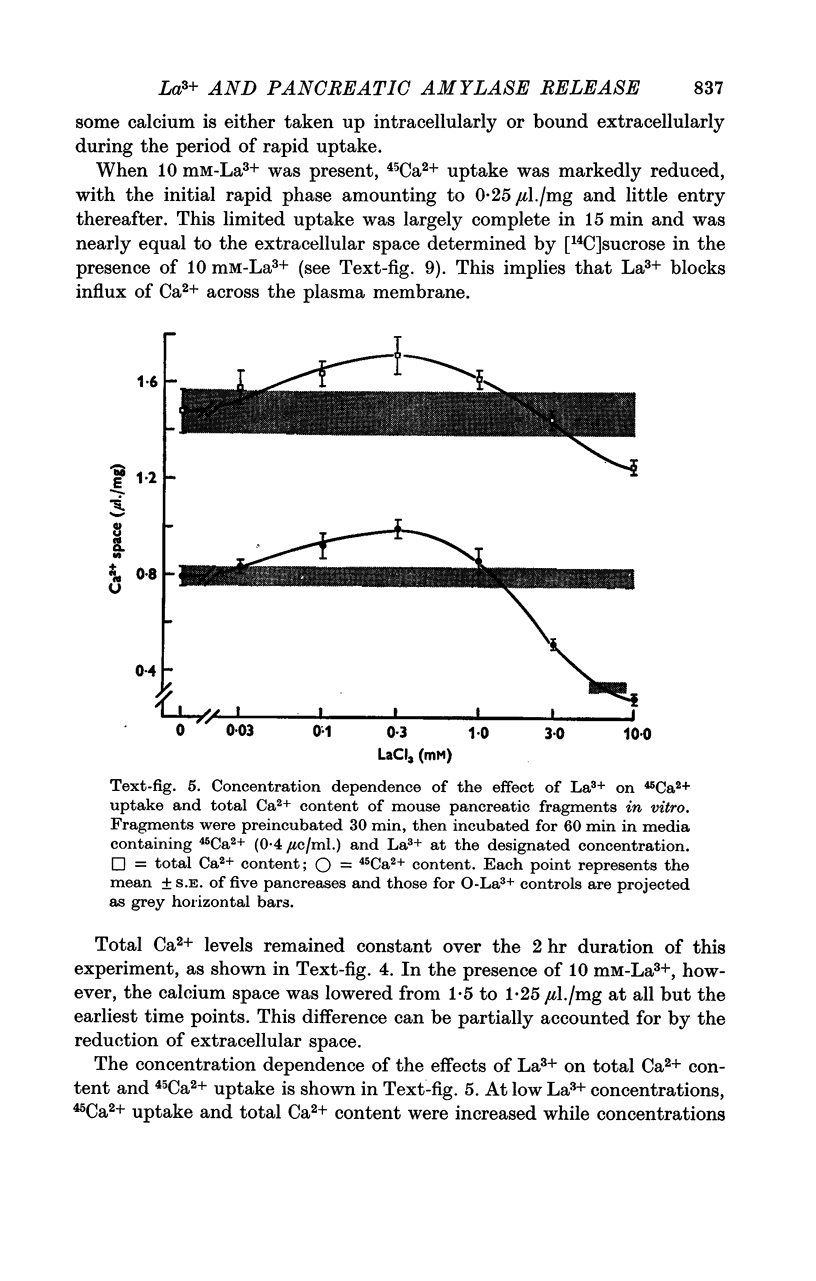
3. Uptake of 45Ca2+ was multiphasic with equilibrium with stable Ca2+ still not complete after 2 hr. La3+ (10 mM) limited uptake of 45Ca2+ to the extracellular space and slightly decreased total Ca2+ content. Lower concentrations of La3+ affected 45Ca2+ uptake and total Ca2+ content in a biphasic manner which paralleled effects on amylase release.
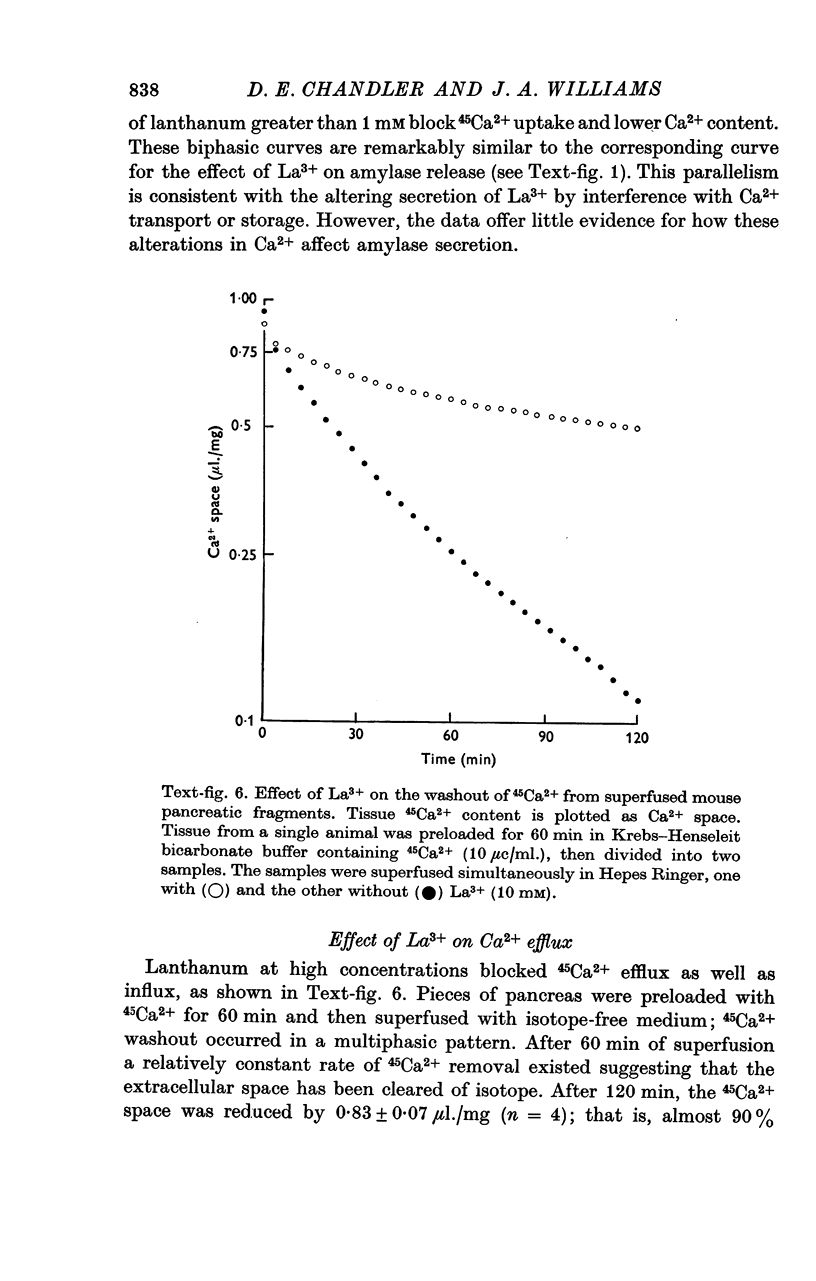
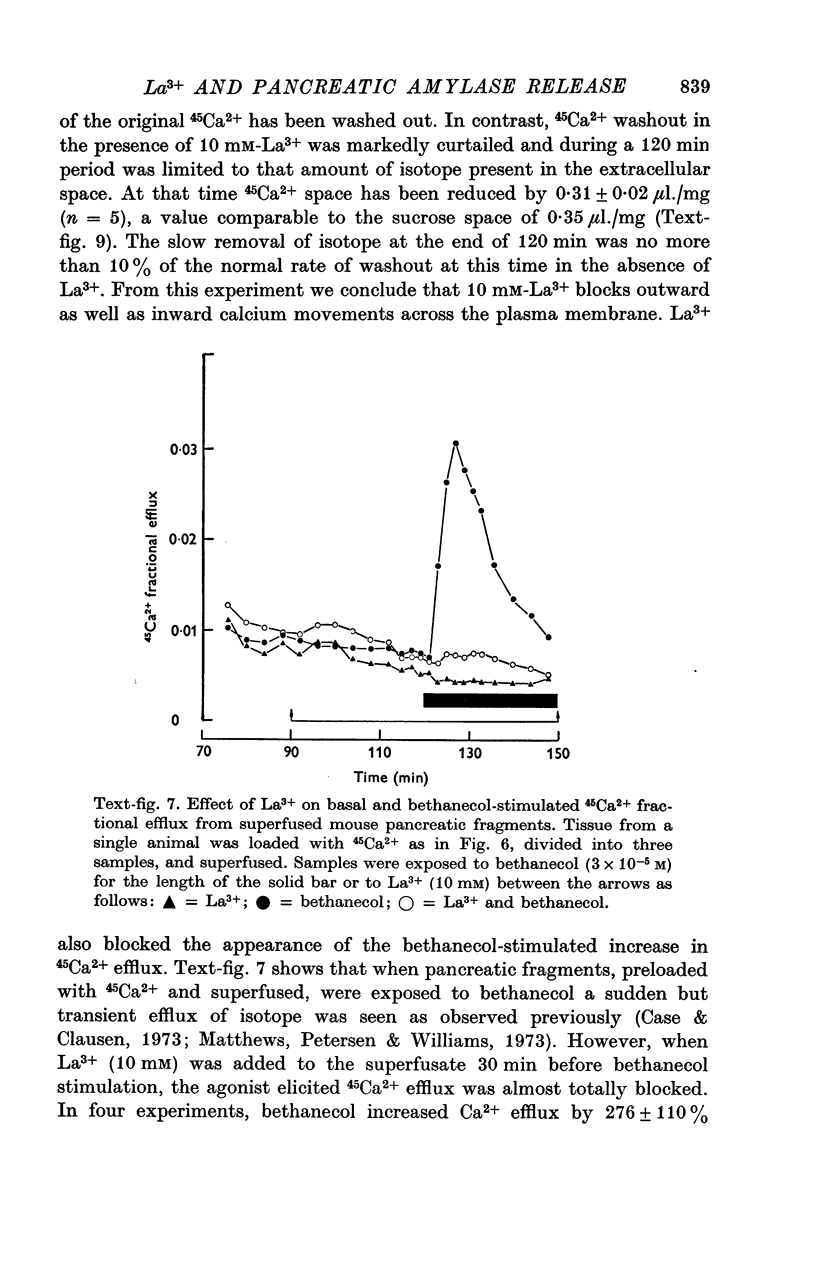
4. La3+ restricted washout of 45Ca2+ to isotope in the extracellular space and abolished the bethanecol-stimulated increase in 45Ca2+ efflux.
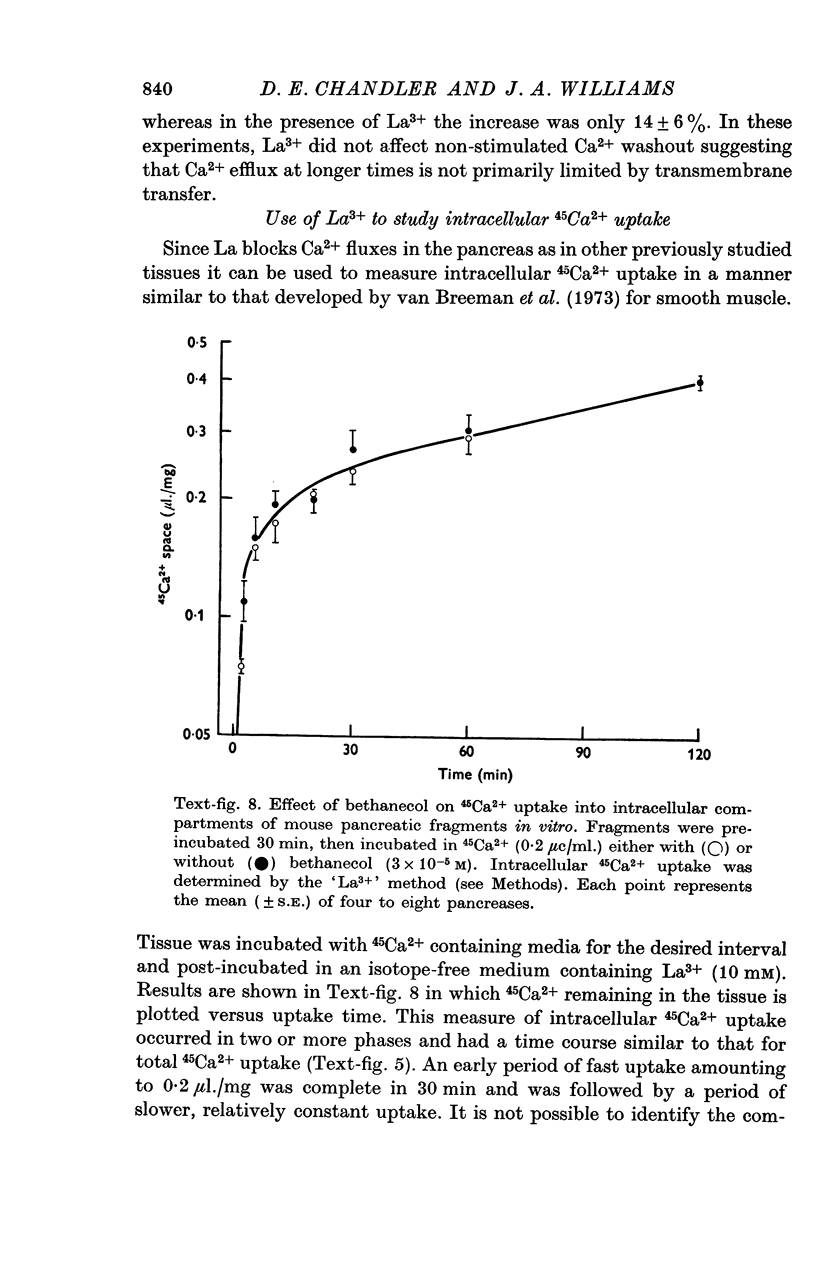
5. Uptake of 45Ca2+ into intracellular space, as measured by the `lanthanum' method, was not affected by bethanecol.
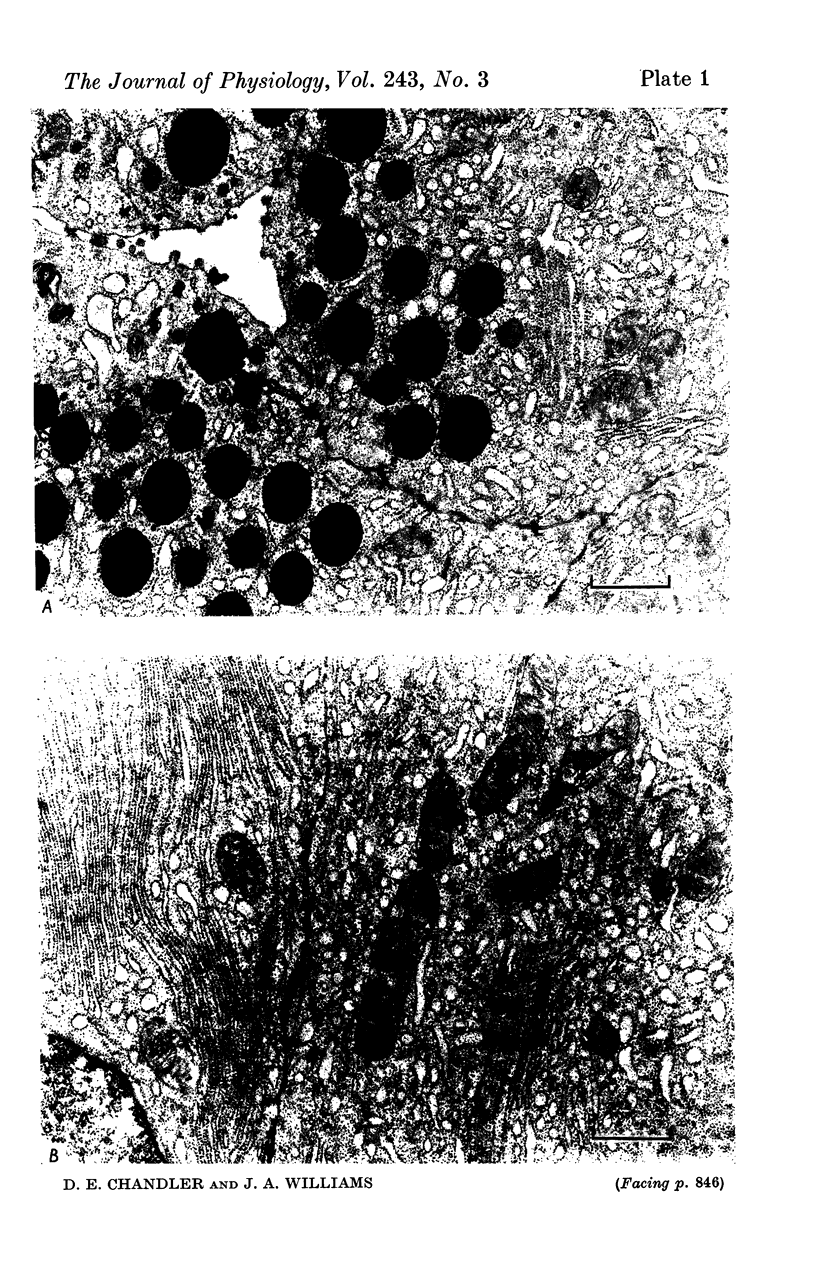
6. Tissue ultrastructure and Na+ and K+ content were not affected by La3+.
7. It is concluded that an influx of extracellular Ca2+ is not important for triggering of secretion and that La3+ may inhibit amylase release by acting on the release process rather than on Ca2+ influx.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S., Fast D., Tenenhouse A. Role of Ca 2+ and cyclic AMP in protein secretion from rat exocrine pancreas. Biochim Biophys Acta. 1972 Oct 25;279(3):561–572. doi: 10.1016/0304-4165(72)90178-x. [DOI] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Frank J. S. Lanthanum in heart cell culture. Effect on calcium exchange correlated with its localization. J Cell Biol. 1972 Sep;54(3):441–455. doi: 10.1083/jcb.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Legros J. J., Grau J. D., Nordmann J. J., Dreifuss J. J. Release of neurohypophysial hormones by exocytosis. Nat New Biol. 1973 Jan 17;241(107):86–88. doi: 10.1038/newbio241086a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Wilding P., Haverback B. J. A new method for the determination of alpha-amylase. Experientia. 1967 Oct 15;23(10):805–805. doi: 10.1007/BF02146851. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Shea S. M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971 Dec;51(3):611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen D., Van Breemen C. Calcium exchange diffusion in a porous phospholipid ion-exchange membrane. Nature. 1969 Aug 30;223(5209):898–900. doi: 10.1038/223898a0. [DOI] [PubMed] [Google Scholar]
- Weiss G. B., Goodman F. R. Effects of lanthanum on contraction, calcium distribution and Ca45 movements in intestinal smooth muscle. J Pharmacol Exp Ther. 1969 Sep;169(1):46–55. [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]