Abstract
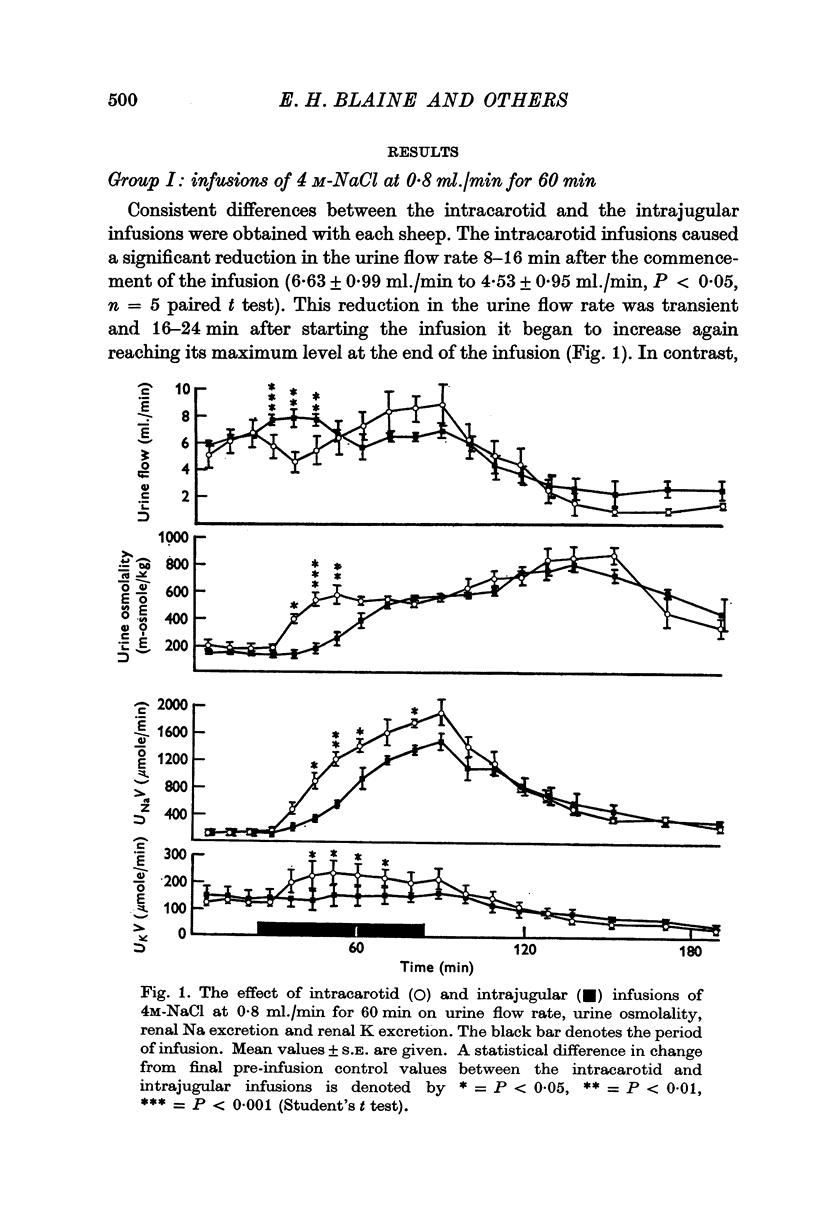
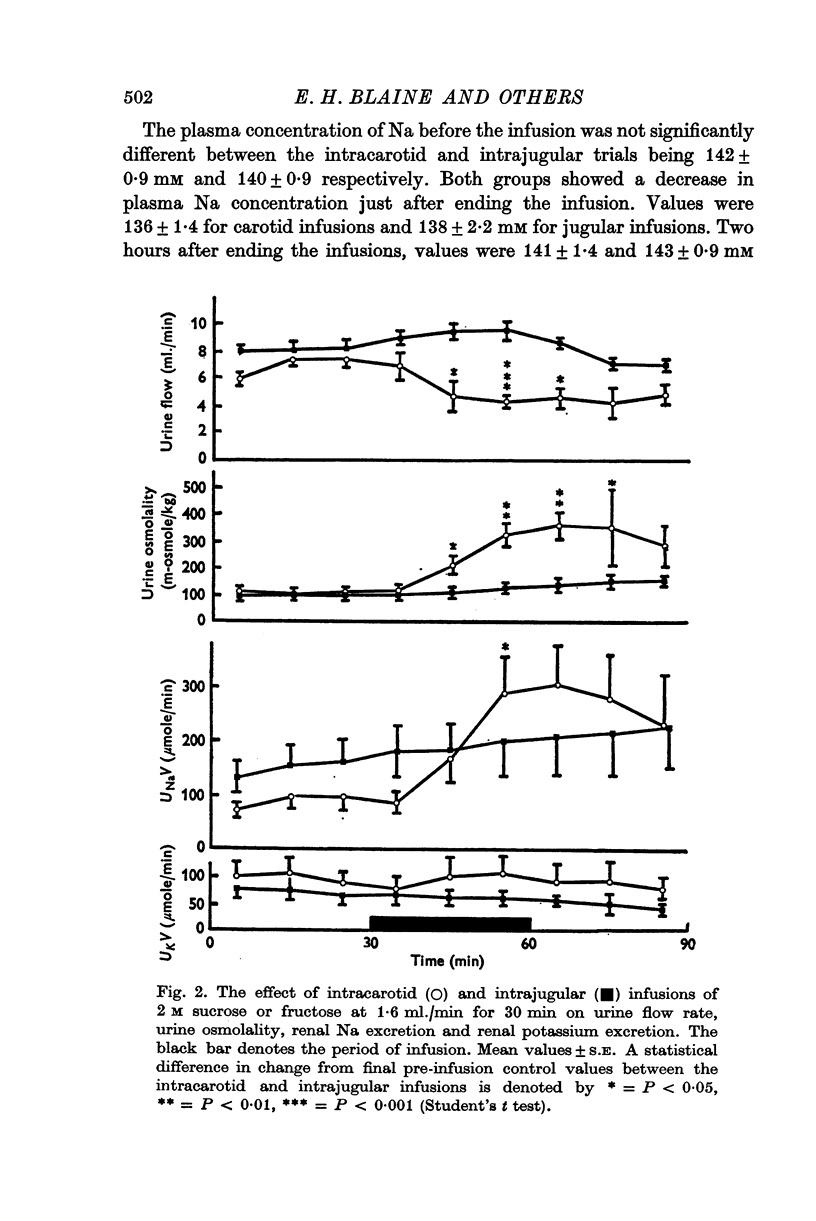
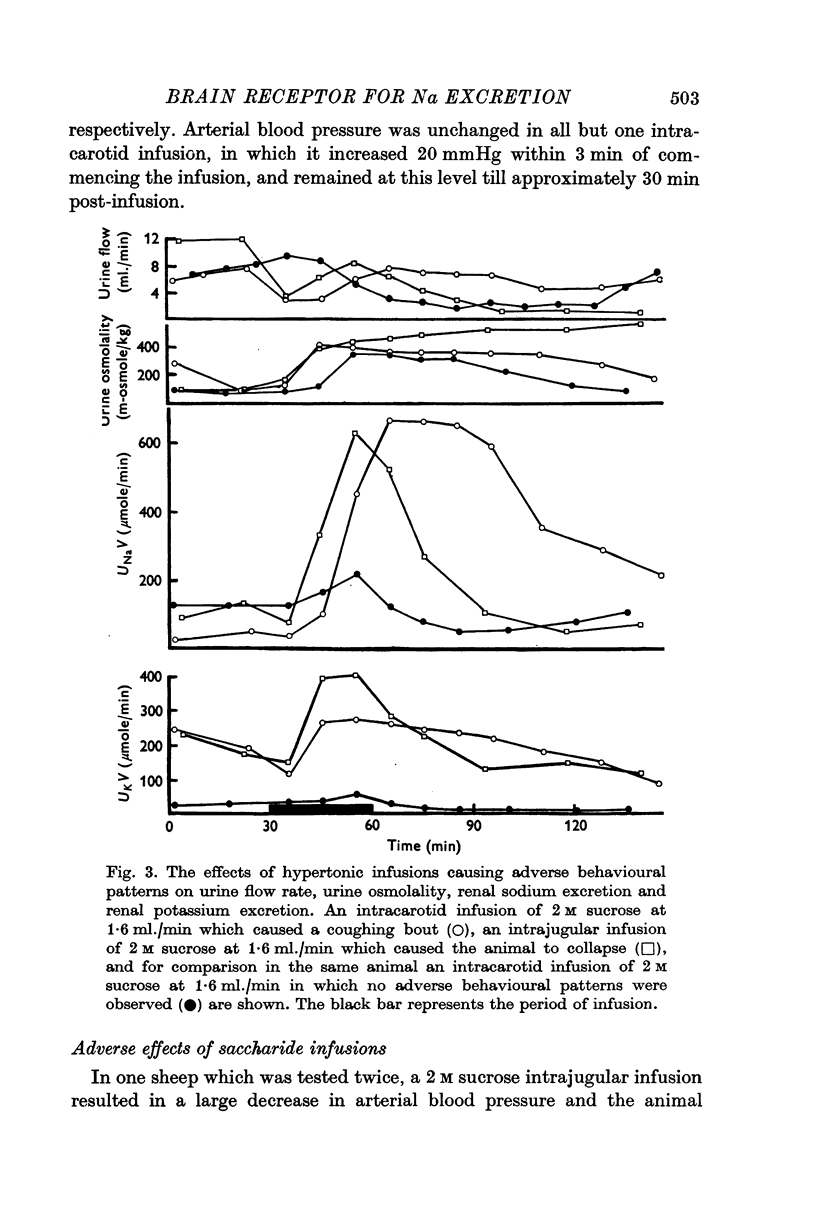
1. The effect on renal Na and water excretion of increasing the NaCl concentration of blood supplying the brain was investigated in conscious water-loaded sheep. Intracarotid infusion ot 4 M-NACl at 0-8 ml./min for 60 min was compared with equivalent intrajugular infusion. 2. A more rapid increase in renal Na excretion and urine osmolality occurred with the intracarotid infusions than with intrajugular infusions. 3. Intracarotid infusions of 2 M sucrose or fructose at 1-6 ml./min for greater increase in renal Na excretion, urine osmolality and a decrease in urine flow rate. 4. The results suggest that there are receptors in the brain sensitive to changes in extracellular tonicity which influence renal Na excretion. It is possible that changes in ADH secretion alone mediate the early natriuresis seen with intracarotid hypertonic infusions although an alternative concurrent mechanism cannot be ruled out.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSLOW W. P., Jr, WESSON L. G., Jr Some effects of pressor-antidiuretic and oxytocic fractions of posterior pituitary extract on sodium, chloride, potassium and ammonium excretion in the dog. Am J Physiol. 1955 Sep;182(3):561–566. doi: 10.1152/ajplegacy.1955.182.3.561. [DOI] [PubMed] [Google Scholar]
- Andersson B., Dallman M. F., Olsson K. Evidence for a hypothalamic control of renal sodium excretion. Acta Physiol Scand. 1969 Mar;75(3):496–510. doi: 10.1111/j.1748-1716.1969.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Andersson B., Jobin M., Olsson K. A study of thirst and other effects of an increased sodium concentration in the 3rd brain ventricle. Acta Physiol Scand. 1967 Jan-Feb;69(1):29–36. doi: 10.1111/j.1748-1716.1967.tb03488.x. [DOI] [PubMed] [Google Scholar]
- BALDWIN B. A., BELL F. R. The anatomy of the cerebral circulation of the sheep and ox. The dynamic distribution of the blood supplied by the carotid and vertebral arteries to cranial regions. J Anat. 1963 Apr;97:203–215. [PMC free article] [PubMed] [Google Scholar]
- BEILHARZ S., BOTT E. A., DENTON D. A., SABINE J. R. THE EFFECT OF INTRACAROTID INFUSIONS OF 4M NAC1 ON THE SODIUM DRINKING OF SHEEP WITH A PAROTID FISTULA. J Physiol. 1965 May;178:80–91. doi: 10.1113/jphysiol.1965.sp007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS F. P., PICKFORD M. The effect of posterior pituitary hormones on the excretion of electrolytes, in dogs. J Physiol. 1958 Aug 6;142(3):468–493. doi: 10.1113/jphysiol.1958.sp006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn J. B., Levine N., Kaley G., Rothballer A. B. Natriuresis induced by injection of hypertonic saline into the third cerebral ventricle of dogs. Proc Soc Exp Biol Med. 1969 May;131(1):240–242. doi: 10.3181/00379727-131-33849. [DOI] [PubMed] [Google Scholar]
- Dorn J., Porter J. C. Diencephalic involvement in sodium excretion in the rat. Endocrinology. 1970 May;86(5):1112–1117. doi: 10.1210/endo-86-5-1112. [DOI] [PubMed] [Google Scholar]
- Dorn J., Rothballer A. B. Essential hypernatremia. The experimental model. Arch Neurol. 1973 Feb;28(2):83–90. doi: 10.1001/archneur.1973.00490200031003. [DOI] [PubMed] [Google Scholar]
- Eriksson L., Fernández O., Olsson K. Differences in the antidiuretic response to intracarotid infusions of various hypertonic solutions in the conscious goat. Acta Physiol Scand. 1971 Dec;83(4):554–562. doi: 10.1111/j.1748-1716.1971.tb05113.x. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W. OSMOTIC CONCENTRATION AND DILUTION OF THE URINE. Am J Med. 1964 May;36:670–685. doi: 10.1016/0002-9343(64)90179-2. [DOI] [PubMed] [Google Scholar]
- Gauer O. H., Henry J. P., Behn C. The regulation of extracellular fluid volume. Annu Rev Physiol. 1970;32:547–595. doi: 10.1146/annurev.ph.32.030170.002555. [DOI] [PubMed] [Google Scholar]
- Humphreys M. H., Friedler R. M., Earley L. E. Natrriuresis produced by vasopressin or hemorrhage during water diuresis in the dog. Am J Physiol. 1970 Sep;219(3):658–665. doi: 10.1152/ajplegacy.1970.219.3.658. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Zehr J. E., Moore W. W. Effects of separate and concurrent osmotic and volume stimuli on plasma ADH in sheep. Am J Physiol. 1970 May;218(5):1273–1280. doi: 10.1152/ajplegacy.1970.218.5.1273. [DOI] [PubMed] [Google Scholar]
- KASTIN A. J., LIPSETT M. B., OMMAYA A. K., MOSER J. M., Jr ASYMPTOMATIC HYPERNATREMIA: PHYSIOLOGICAL AND CLINICAL STUDY. Am J Med. 1965 Feb;38:306–315. doi: 10.1016/0002-9343(65)90185-3. [DOI] [PubMed] [Google Scholar]
- KEELER R. Effect of hypothalamic lesions on renal excretion of sodium. Am J Physiol. 1959 Oct;197:847–849. doi: 10.1152/ajplegacy.1959.197.4.847. [DOI] [PubMed] [Google Scholar]
- Macfarlane W. V., Kinne R., Walmsley C. M., Siebert B. D., Peter D. Vasopressins and the increase of water and electrolyte excretion by sheep, cattle and camels. Nature. 1967 Jun 3;214(5092):979–981. doi: 10.1038/214979a0. [DOI] [PubMed] [Google Scholar]
- Moran W. H., Jr, Zimmermann B. Mechanisms of antidiuretic hormone (ADH) control of importance to the surgical patient. Surgery. 1967 Oct;62(4):639–644. [PubMed] [Google Scholar]
- Mouw D. R., Abraham S. F., Blair-West J. R., Coghlan J. P., Denton D. A., McKenzie J. S., McKinley M. J., Scoggins B. A. Brain receptors, renin secretion, and renal sodium retention in conscious sheep. Am J Physiol. 1974 Jan;226(1):56–62. doi: 10.1152/ajplegacy.1974.226.1.56. [DOI] [PubMed] [Google Scholar]
- Mouw D. R., Vander A. J. Evidence for brain Na receptors controlling renal Na excretion and plasma renin activity. Am J Physiol. 1970 Sep;219(3):822–832. doi: 10.1152/ajplegacy.1970.219.3.822. [DOI] [PubMed] [Google Scholar]
- Novakova A., Stevenson J. A. Effect of posterior hypothalamic lesions on renal function in the rat. Can J Physiol Pharmacol. 1971 Nov;49(11):941–950. doi: 10.1139/y71-131. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., WAGNER H. N., Jr, DAVIDSON D. G. The effect of variations in solute excretion and vasopressin dosage on the excretion of water in the dog. J Clin Invest. 1958 Mar;37(3):458–464. doi: 10.1172/JCI103625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson K. Further evidence for the importance of CSF Na+ concentration in central control of fluid balance. Acta Physiol Scand. 1973 Jun;88(2):183–188. doi: 10.1111/j.1748-1716.1973.tb05445.x. [DOI] [PubMed] [Google Scholar]
- Olsson K., McDonald I. R. Lack of antidiuretic response to osmotic stimuli in the early stages of a water diuresis in sheep. J Endocrinol. 1970 Oct;48(2):301–302. doi: 10.1677/joe.0.0480301. [DOI] [PubMed] [Google Scholar]
- Olsson K. On the importance of CSF Na + concentration in central control of fluid balance. Life Sci I. 1972 Apr 15;11(8):397–402. doi: 10.1016/0024-3205(72)90049-5. [DOI] [PubMed] [Google Scholar]
- Perlmutt J. H. Contribution of carotid and vagal reflex mechanisms. Fed Proc. 1968 Sep-Oct;27(5):1149–1155. [PubMed] [Google Scholar]
- Pleasure D., Goldberg M. Neurogenic hypernatremia. Arch Neurol. 1966 Jul;15(1):78–87. doi: 10.1001/archneur.1966.00470130082009. [DOI] [PubMed] [Google Scholar]
- Rabinowitz L., Gunther R. A. Renal concentrating ability in sheep during urea, mannitol, and methylurea diuresis. Am J Physiol. 1972 Apr;222(4):801–806. doi: 10.1152/ajplegacy.1972.222.4.801. [DOI] [PubMed] [Google Scholar]
- SAWYER W. H. Posterior pituitary extracts and excretion of electrolytes by the rat. Am J Physiol. 1952 Jun;169(3):583–587. doi: 10.1152/ajplegacy.1952.169.3.583. [DOI] [PubMed] [Google Scholar]
- SMITH H. W. Salt and water volume receptors: an exercise in physiologic apologetics. Am J Med. 1957 Oct;23(4):623–652. doi: 10.1016/0002-9343(57)90232-2. [DOI] [PubMed] [Google Scholar]
- Thornborough J. R., Passo S. S., Rothballer A. B. Forebrain lesion blockade of the natriuretic response to elevated carotid blood sodium. Brain Res. 1973 Aug 30;58(2):355–363. doi: 10.1016/0006-8993(73)90007-3. [DOI] [PubMed] [Google Scholar]
- Thornborough J. R., Passo S. S., Rothballer A. B. Receptors in cerebral circulation affecting sodium excretion in the cat. Am J Physiol. 1973 Jul;225(1):138–141. doi: 10.1152/ajplegacy.1973.225.1.138. [DOI] [PubMed] [Google Scholar]
- VERNEY E. B. Renal excretion of water and salt. Lancet. 1957 Dec 28;273(7009):1295–1298. doi: 10.1016/s0140-6736(57)91635-5. [DOI] [PubMed] [Google Scholar]


