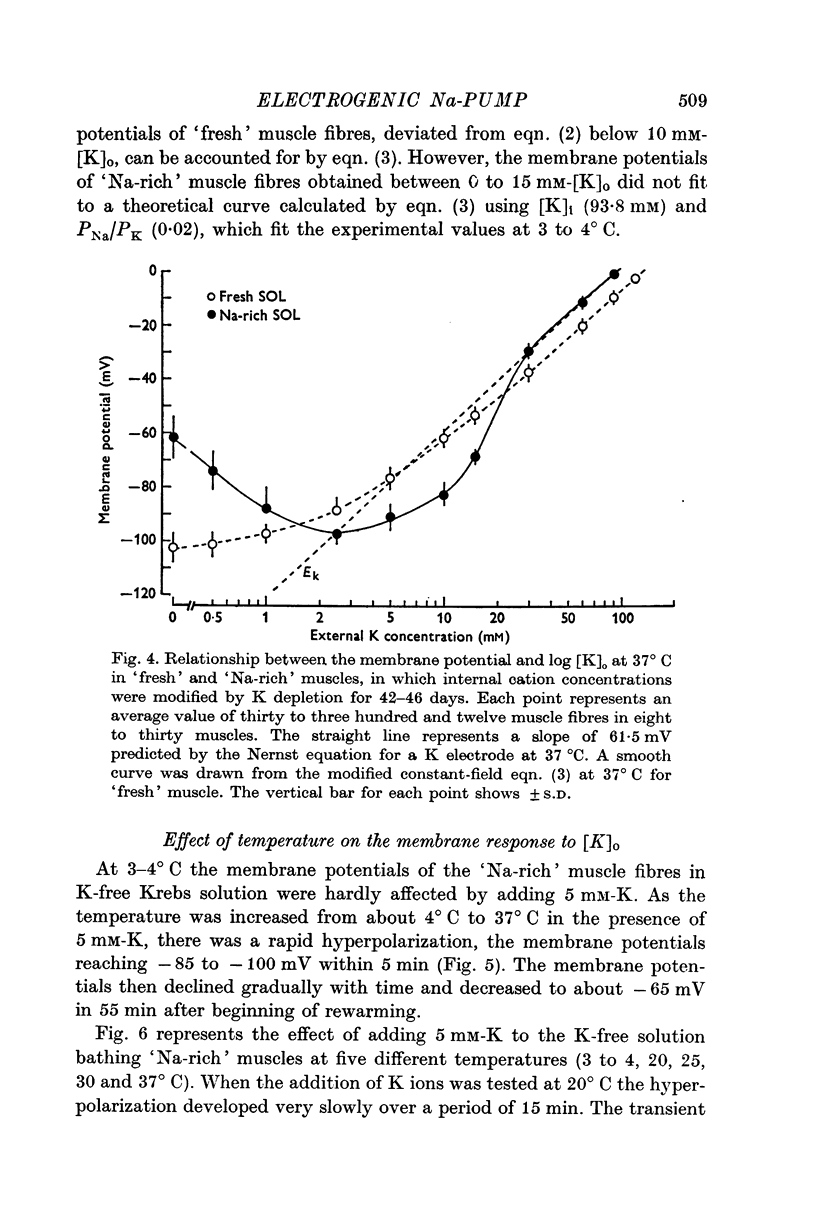
Abstract
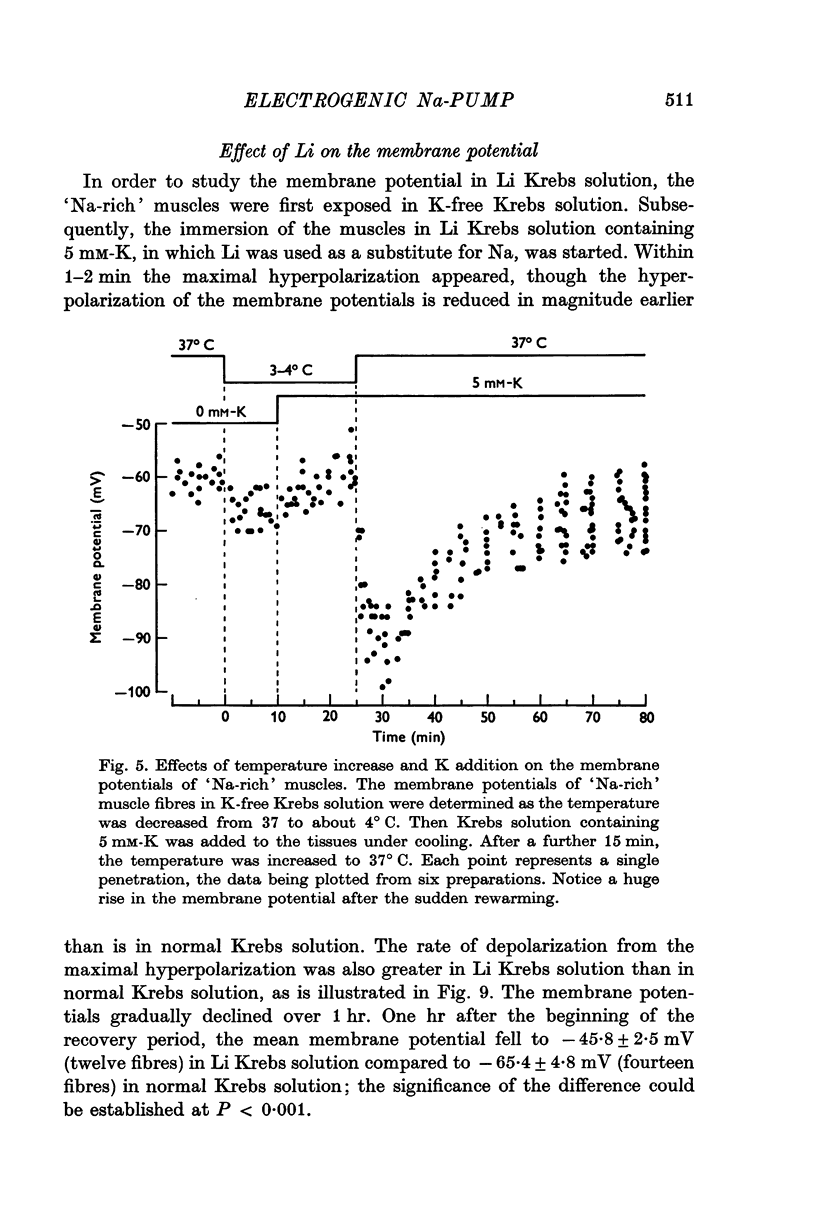
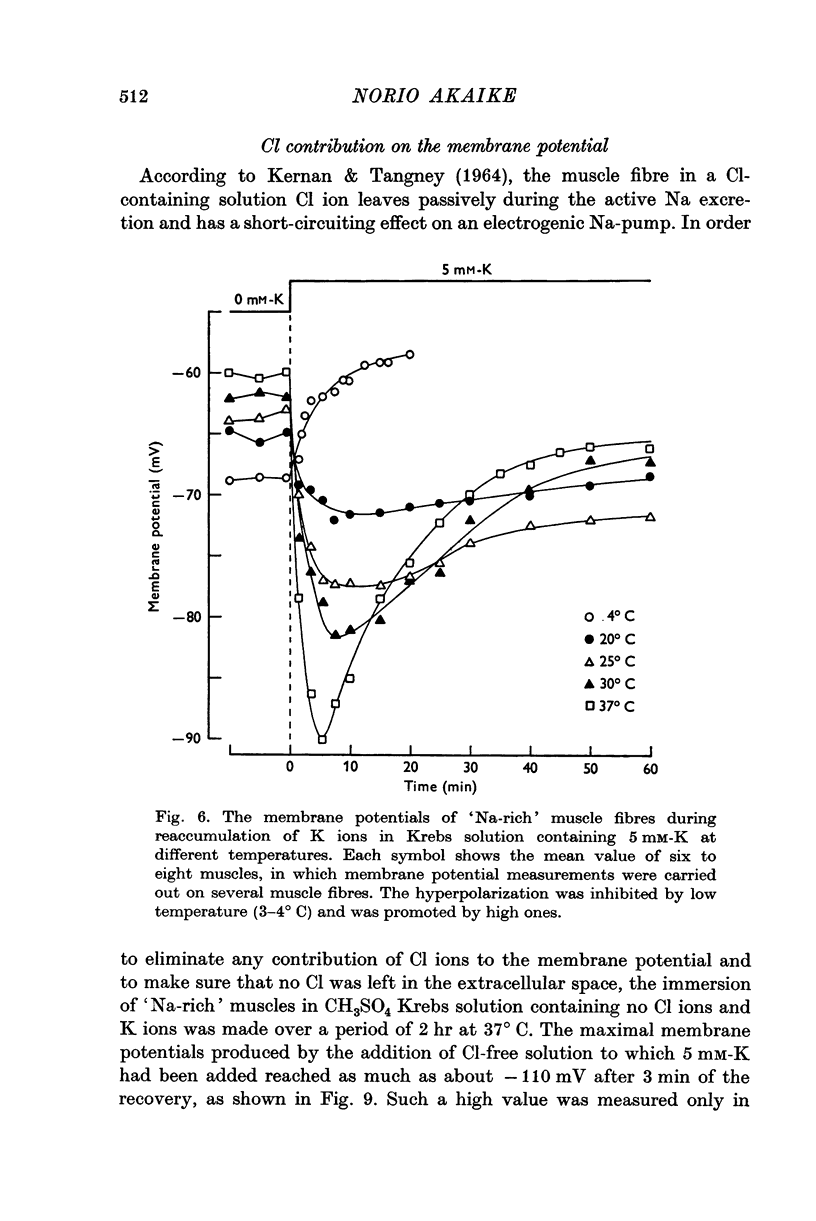
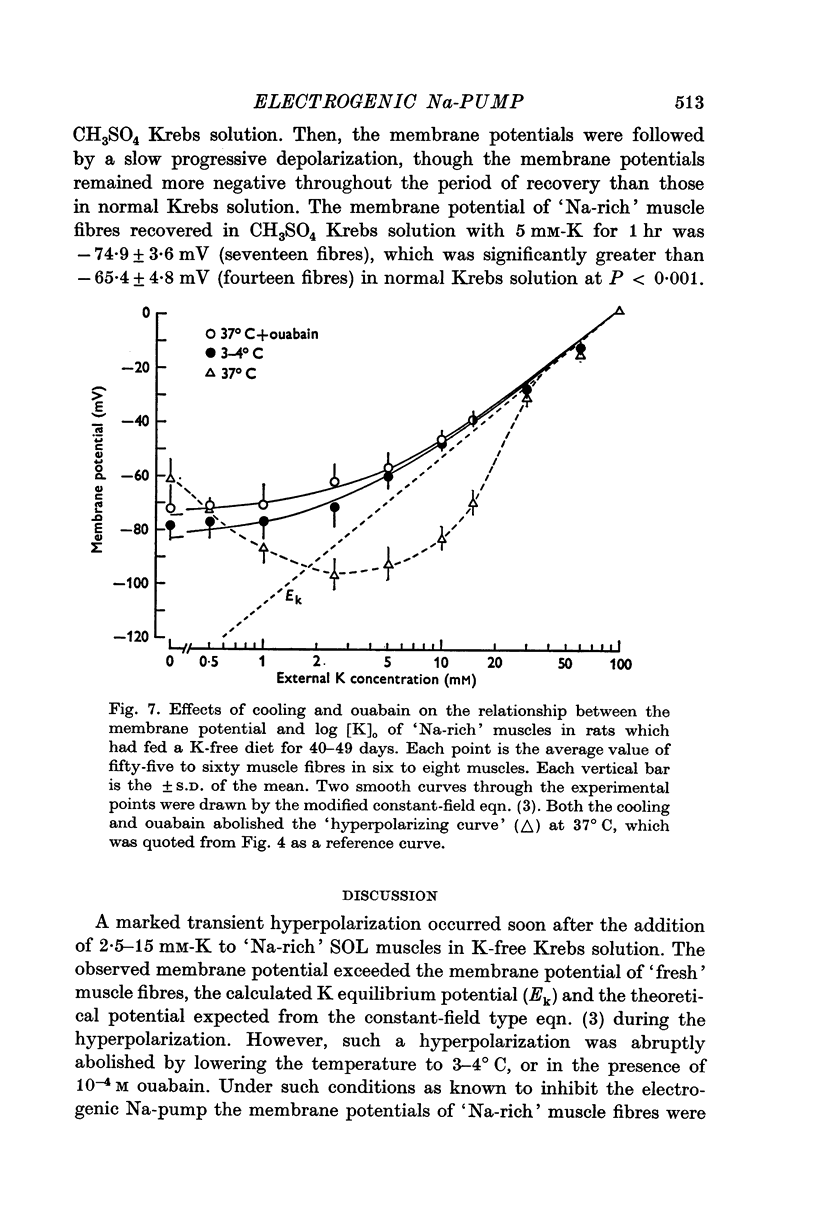
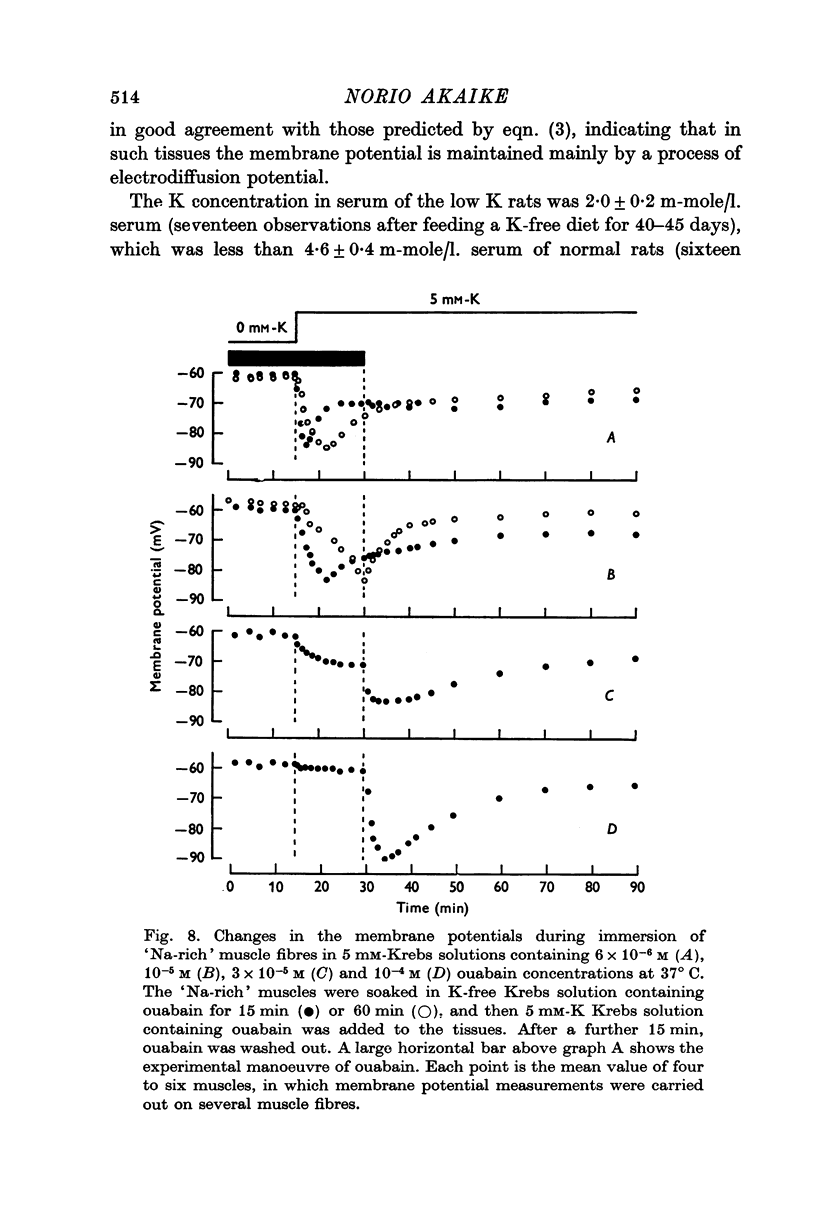
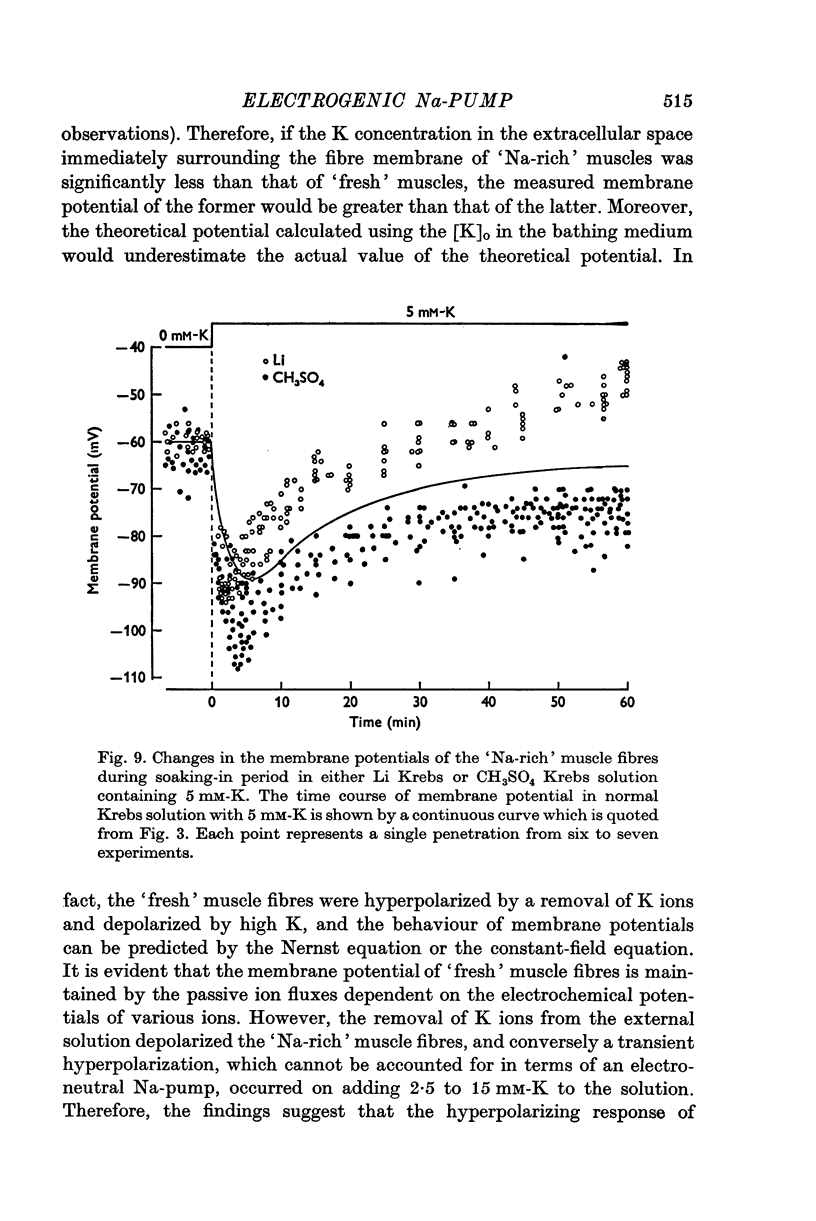
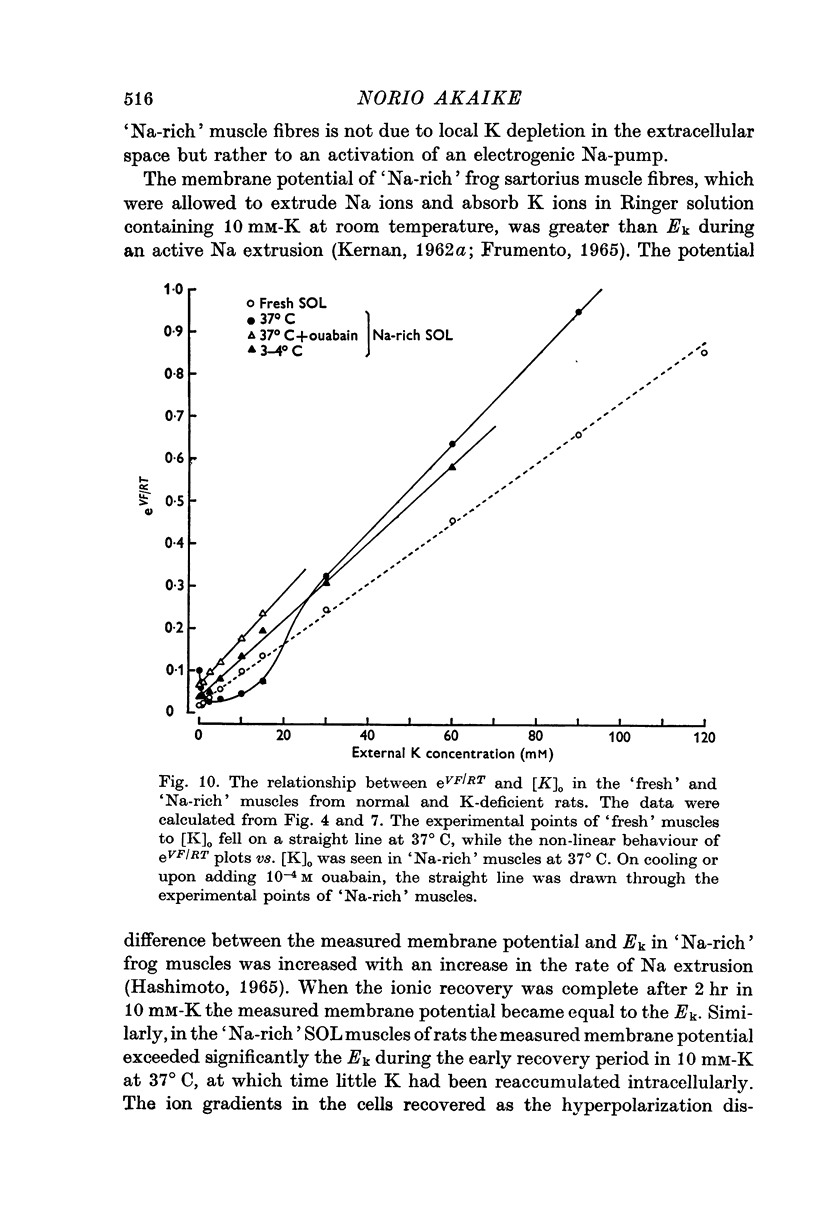
1. Relationship between the resting membrane potential and the changes in the intraceullar Na and K concentrations ([Na]i and [K]i) was studied in 'Na-loaded' and K-depleted' soleus (SOL) muscles of rats which had fed a K-free diet for 40 and more days. 2. The extracellular space of the muscles was not significantly different between normal and K-deficient rats. The inulin space in both the 'fresh' and Na-rich' muscles can be determined by the same function relating the space to the muscle weight. 3. Presence of 2-5-15 mM-K in the recovery solution hyperpolarized the 'Na-rich' muscul fibres at the beginning of recovery. The hyperpolarized membrane potential exceeded, beyond the measured potential of 'fresh' muscle fibres, the theoretical potential derived from the ionic theory, or even beyond Ek. Then, the measured membrane potential declined progressively during the immersion in a recovery solution and returned to the steady-state value When a considerable Na extrusion and K uptake took place, the measured membrane potential became equal to Ek. 4.he maximal hyperpolarization occurring immediately after immersion in the recovery solution became smaller and had a shorter duration when increasing the external K concentration ([K]o) from 2-5 to 15mM. 5. The K-sensitive hyperpolarization was completely abolished on exposure to 0mM [K]o, on cooling to ca. 4 degrees C, and in the presence of oubain (10(-4) M). The inhibitory effects were reversed on returning to the control conditions. The membrane potential obtained after inhibition of the electrogenic Na-pump with cooling or ouabain agrees well with that predicted by the 'constant-field' equation. 7. The external Cl ions had a short-circuiting effect on the electrogenic Na-pumping activated on adding K ions. 8. The replacement of Na ions in a recovery solution with Li ions resulted in a faster rate of depolarization from the maximal hyperpolarizationp. It is concluded that the resting membrane potential of 'Na-loaded' and 'K-depleted' SOL muscle fibres is the sum of an ionic diffusion potential predicted by either the Nernst equation or the constant-field equation and of the potential produced by an electrogenic Na-pump.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N. Cation concentration change in rat skeletal muscles associated with potassium deficiency and denervation. Jpn J Physiol. 1969 Aug;19(4):420–438. doi: 10.2170/jjphysiol.19.420. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. E. INFLUENCE OF LITHIUM IONS ON THE TRANSMEMBRANE POTENTIAL AND CATION CONTENT OF CARDIAC CELLS. J Gen Physiol. 1964 Jan;47:501–530. doi: 10.1085/jgp.47.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Hingerty D. Relations between potassium and sodium levels in mammalian muscle and blood plasma. Biochem J. 1948;42(3):372–376. doi: 10.1042/bj0420372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockry M., Kernan R. P., Tangney A. Active transport of sodium and potassium in mammalian skeletal muscle and its modification by nerve and by cholinergic and adrenergic agents. J Physiol. 1966 Sep;186(1):187–200. doi: 10.1113/jphysiol.1966.sp008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S. SODIUM PUMP: ITS ELECTRICAL EFFECTS IN SKELETAL MUSCLE. Science. 1965 Mar 19;147(3664):1442–1443. doi: 10.1126/science.147.3664.1442. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Ochs S. Effects of sodium extrusion and local anaesthetics on muscle membrane resistance and potential. J Physiol. 1966 Nov;187(1):5–21. doi: 10.1113/jphysiol.1966.sp008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y. Resting potentials of Na-loaded sartorius muscle fibres of toads during recovery in high K ringer. Kumamoto Med J. 1965 Mar 31;18(1):23–30. [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. The role of lactate in the active excretion of sodium by frog muscle. J Physiol. 1962 Jun;162:129–137. doi: 10.1113/jphysiol.1962.sp006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Yonemura K. The extracellular space in red and white muscles of the rat. Jpn J Physiol. 1967 Dec 15;17(6):698–707. doi: 10.2170/jjphysiol.17.698. [DOI] [PubMed] [Google Scholar]
- MUNTWYLER E., GRIFFIN G. E. Effect of potassium on electrolytes of rat plasma and muscle. J Biol Chem. 1951 Dec;193(2):563–573. [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SRETER F. A., WOO G. CELL WATER, SODIUM, AND POTASSIUM IN RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1290–1294. doi: 10.1152/ajplegacy.1963.205.6.1290. [DOI] [PubMed] [Google Scholar]
- Sato M., Akaike N., Nishi R. Membrane potentials of frog sartorius muscle fibers, in which potassium ions were replaced by sodium. Kumamoto Med J. 1967 Mar 31;20(1):39–55. [PubMed] [Google Scholar]
- Sokolove P. G., Cooke I. M. Inhibition of impulse activity in a sensory neuron by an electrogenic pump. J Gen Physiol. 1971 Feb;57(2):125–163. doi: 10.1085/jgp.57.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura K., Sato M. The resting membrane potential and cation movement in frog muscle fibers after exposure to lithium ions. Jpn J Physiol. 1967 Dec 15;17(6):678–697. doi: 10.2170/jjphysiol.17.678. [DOI] [PubMed] [Google Scholar]


