Abstract
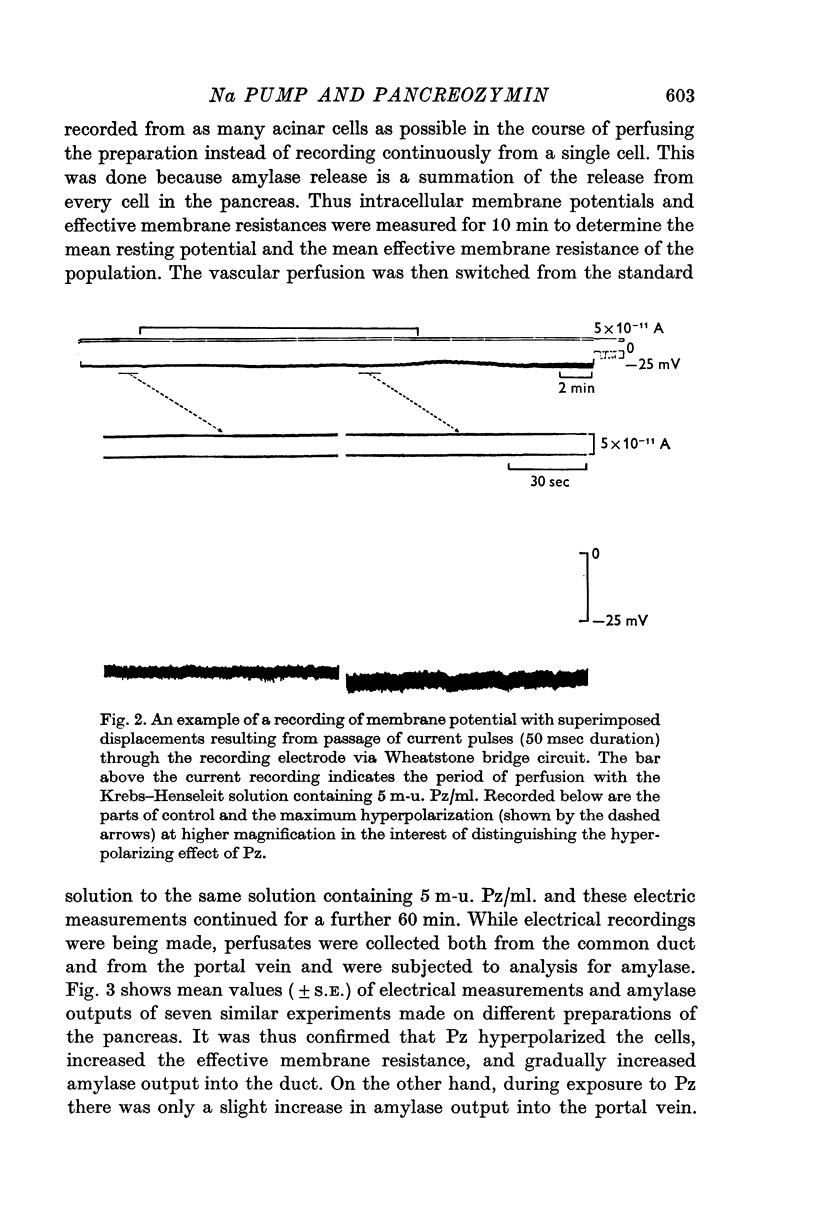
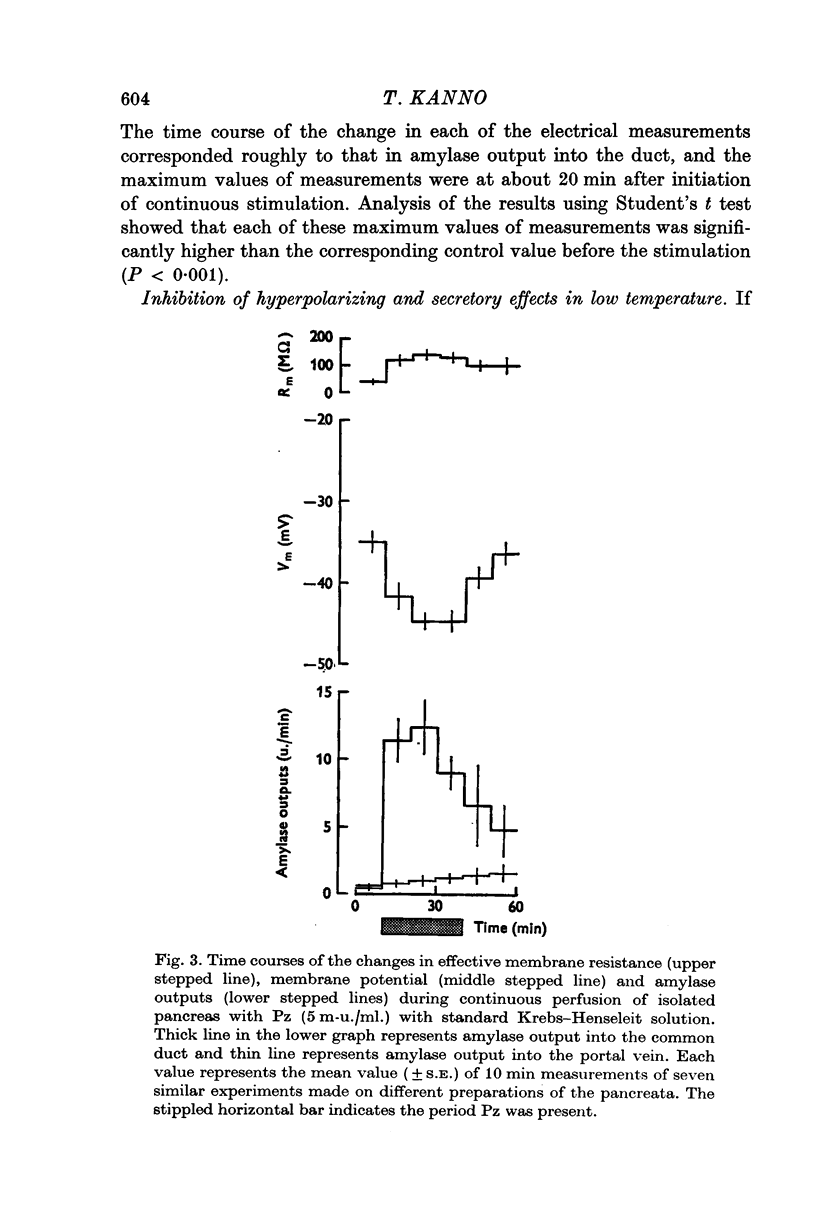
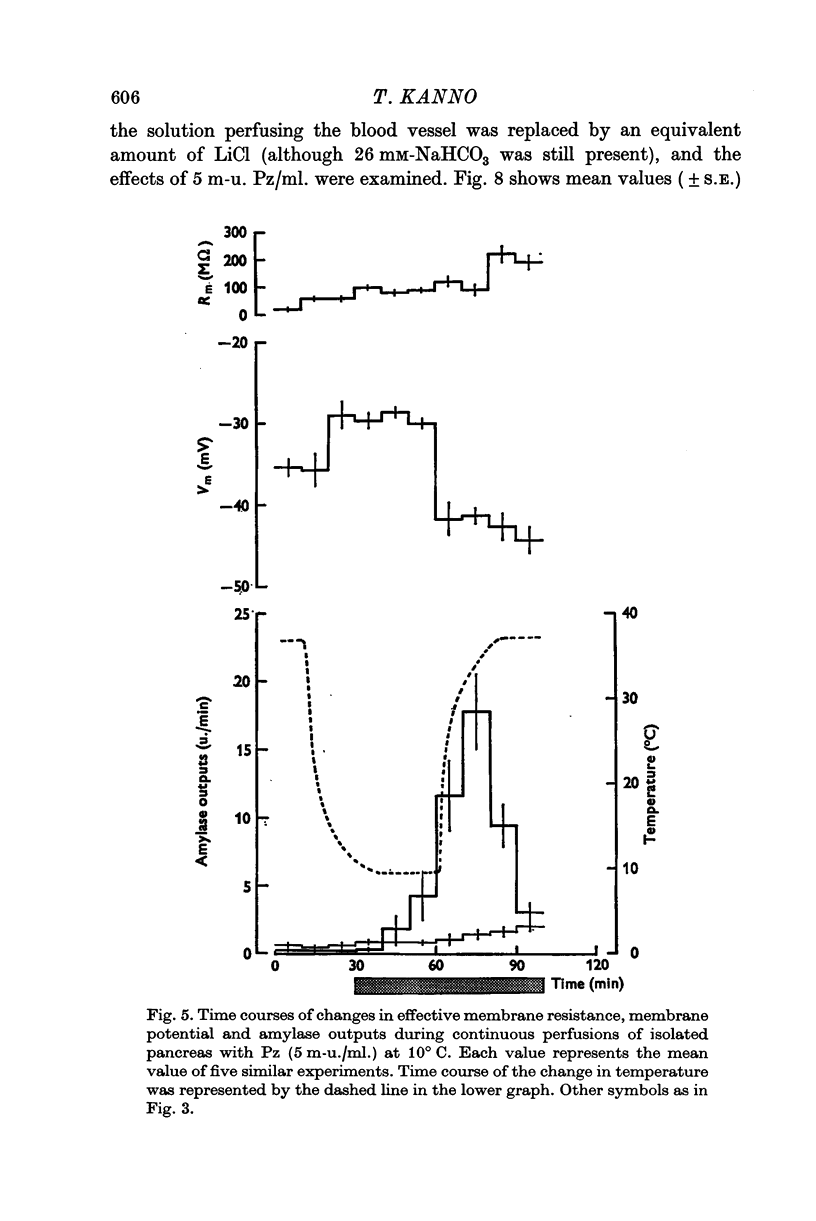
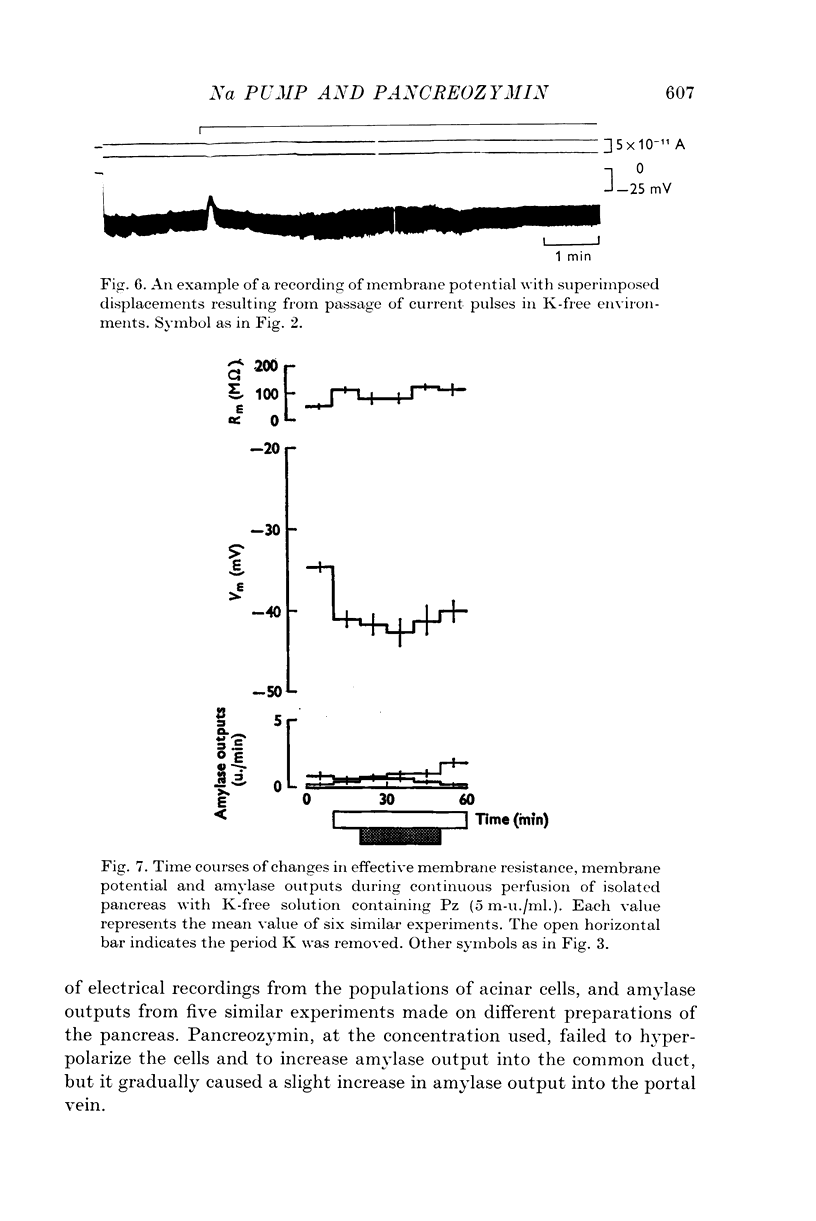
1. Transmembrane potential, effective membrane resistance, and amylase release were recorded simultaneously from acinar cells of isolated rat pancreas perfused with Krebs-Henseleit solution. 2. The hyperpolarizing effect of pancreozymin (Pz) was confirmed by perfusing with a solution containing 5 m-u. Pz/ml. 3. The hyperpolarizing effect of Pz disappeared in the following environments: (a) in low ambient temperature, (b) in K-free medium, (c) in low Na medium, and (d) in the presence of ouabain. In these environments, transient depolarization was frequently observed immediately after stimulation. It is suggested that there are two components in the effect of Pz on the membrane potential of the acinar cells: transient depolarization which coincides with an increase in Na permeability in the initial phase, and continuous hyperpolarization due to an electrogenic Na pump which conceals transient depolarizing phase when the pump is dominant. 4. The secretory effect of Pz was inhibited under conditions that suppressed the electrogenic Na pump. It is proposed that the Na pump activated by Pz maintains the passive Na-influx, increases [Ca2+]i, and, in consequence, uphold the amylase output during continuous stimulation. 5. A medium which was used to bathe the sectioned pancreas with Pz was found to contain a substance which depolarized the acinar cells.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRICK J., HARPER A. A., RAPER H. S. On the preparation of secretin and pancreozymin. J Physiol. 1949 Dec;110(3-4):367–376. doi: 10.1113/jphysiol.1949.sp004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M. Calcium and gastrointestinal secretion. Digestion. 1973;8(3):269–288. doi: 10.1159/000197324. [DOI] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch U. R., Wormsley K. G. Pancreatic enzyme response to secretin and cholecystokinin-pancreozymin in the rat. J Physiol. 1973 Oct;234(1):79–94. doi: 10.1113/jphysiol.1973.sp010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebell H., Steffen C., Bode C. Stimulatory effect of pancreozymin-cholecystokinin on calcium secretion in pancreatic juice of dogs. Gut. 1972 Jun;13(6):477–482. doi: 10.1136/gut.13.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S., Grondin G. Effect of lanthanum on 45Ca flux and secretion of protein from rat exocrine pancreas. Life Sci. 1973 Oct 1;13(7):783–794. doi: 10.1016/0024-3205(73)90069-6. [DOI] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Leroy J., Morisset J. A., Webster P. D. Dose-related response of pancreatic synthesis and secretion to cholecystokinin-pancreazymin. J Lab Clin Med. 1971 Jul;78(1):149–157. [PubMed] [Google Scholar]
- Lin K. T., Johnstone R. M. Active transport of glycine by mouse pancreas. Evidence against the Na + gradient hypothesis. Biochim Biophys Acta. 1971 Oct 12;249(1):144–158. doi: 10.1016/0005-2736(71)90091-5. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. The ionic dependence of the membrane potential and acetylcholine-induced depolarization of pancreatic acinar cells. J Physiol. 1972 Oct;226(2):98P–99P. [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrogenic sodium pump in pancreatic acinar cells. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):115–119. doi: 10.1098/rspb.1973.0037. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Matthews E. K. The effect of pancreozymin and acetylcholine on the membrane potential of the pancreatic acinar cells. Experientia. 1972 Sep 15;28(9):1037–1038. doi: 10.1007/BF01918657. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Saito A., Kanno T. Concentration of pancreozymin as a determinant of the exocrine-endocrine partition of pancreatic enzymes. Jpn J Physiol. 1973 Oct;23(5):477–495. doi: 10.2170/jjphysiol.23.477. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The role of calcium in neurohumoral and neurohormonal extrusion processes. J Pharm Pharmacol. 1968 Dec;20(12):889–910. doi: 10.1111/j.2042-7158.1968.tb09672.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]


