Abstract
1. The electrical properties of horizontal cells in the mudpuppy in light and dark were measured with a pair of micropipettes separated by about 1 mum with low coupling resistance so that no bridge circuitry was required. 2. All horizontal cells studied showed significant anomalous rectification: the current-voltage characteristic for about 60 per cent of the cells studied had a slope resistance of about 20-30 M omega at the dark potential level; the slope resistance increased by about 15% for each 10 mV depolarization and decreased by about 15% for each 10 mV hyperpolarization. The remaining 40% of the horizontal cells showed a higher input resistance at corresponding potential levels but had similar rectifying properties. 3. The increase in resistance with depolrization developed with a time course of about 1/2 sec when steady steps of outward current were passed across the membrane, but the time course for resistance decrease with hyperpolarization was much shorter for steady inward current steps. In about half the horizontal cells there was a transient decrease in resistance lasting about 100 msec immediately following the outward current steps superimposed upon the slower sustained resistance increase. 4. The normal 20-30 mV hyperpolarizing light response was associated with little or no change in input resistance. However, if the membrane potential was held at the dark potential level with extrinsic current, thereby eliminating the potential-dependent resistance change, a light-elicited resistance increase of about 10 M omega was measured. 5. The time-dependent change in membrane resistance elicited by polarizing steps of current obscured the reversal potential for the response. However, when the reversal potential was measured at short times following polarization of the membrane, before the time-dependent resistance change developed, it was estimated at between +15 and +50 m V. 6. The results suggest that the horizontal cell response is mediated by a light-elicited resistance increase at the synaptic membrane which is obscured by a potential- and time-dependent resistance decrease at another part of the membrane.
Full text
PDF



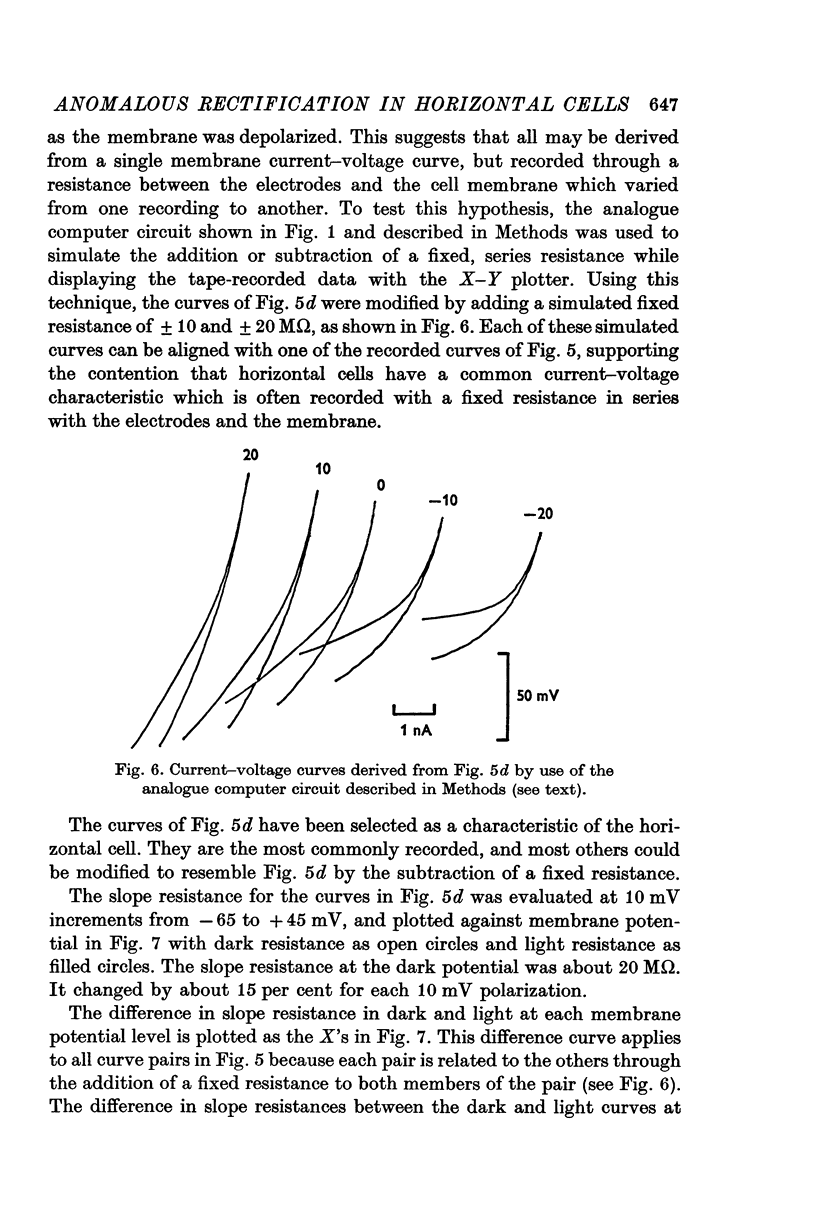
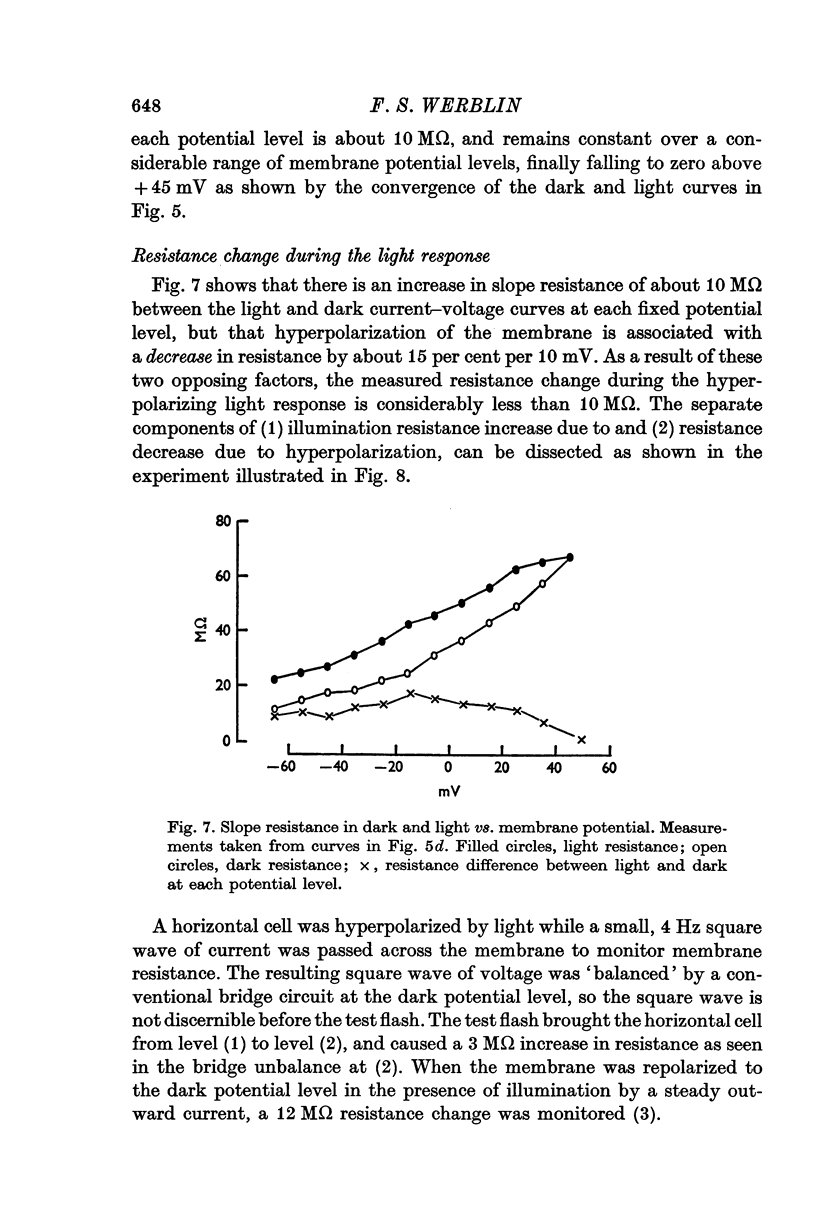
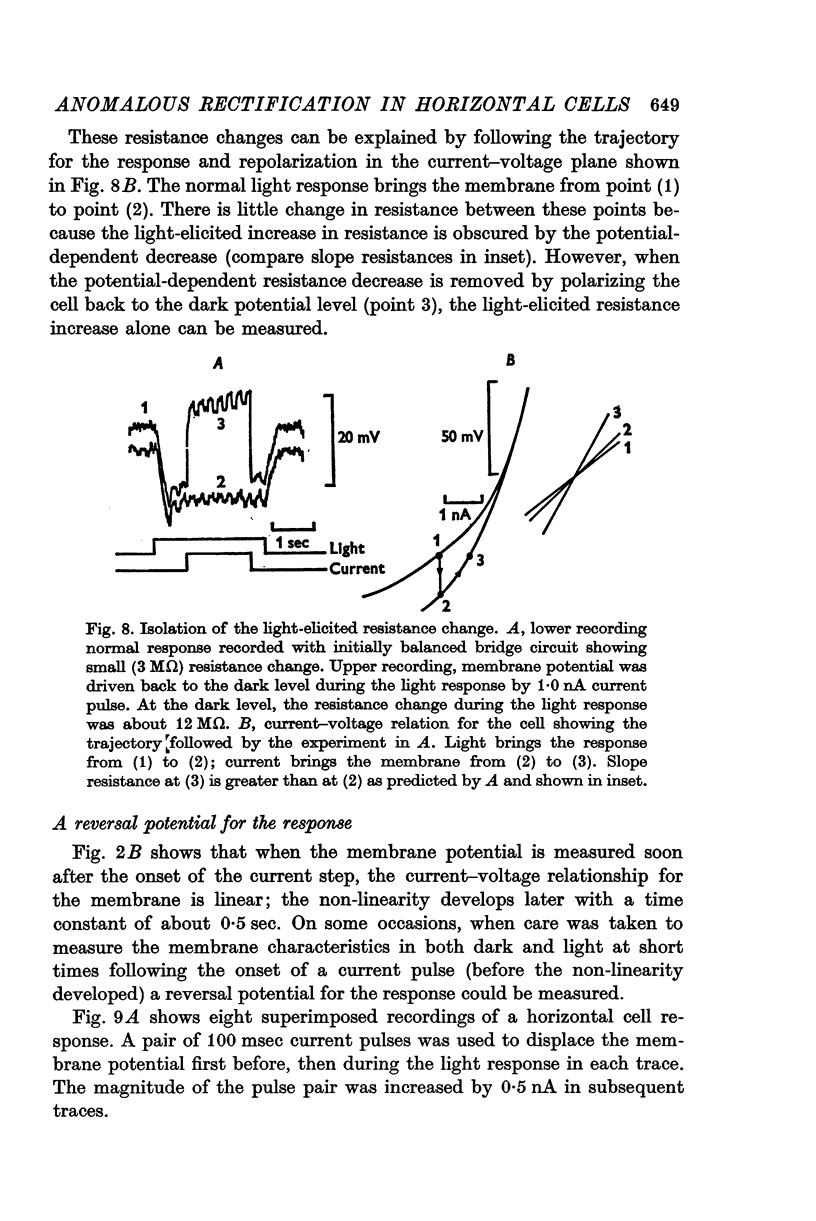
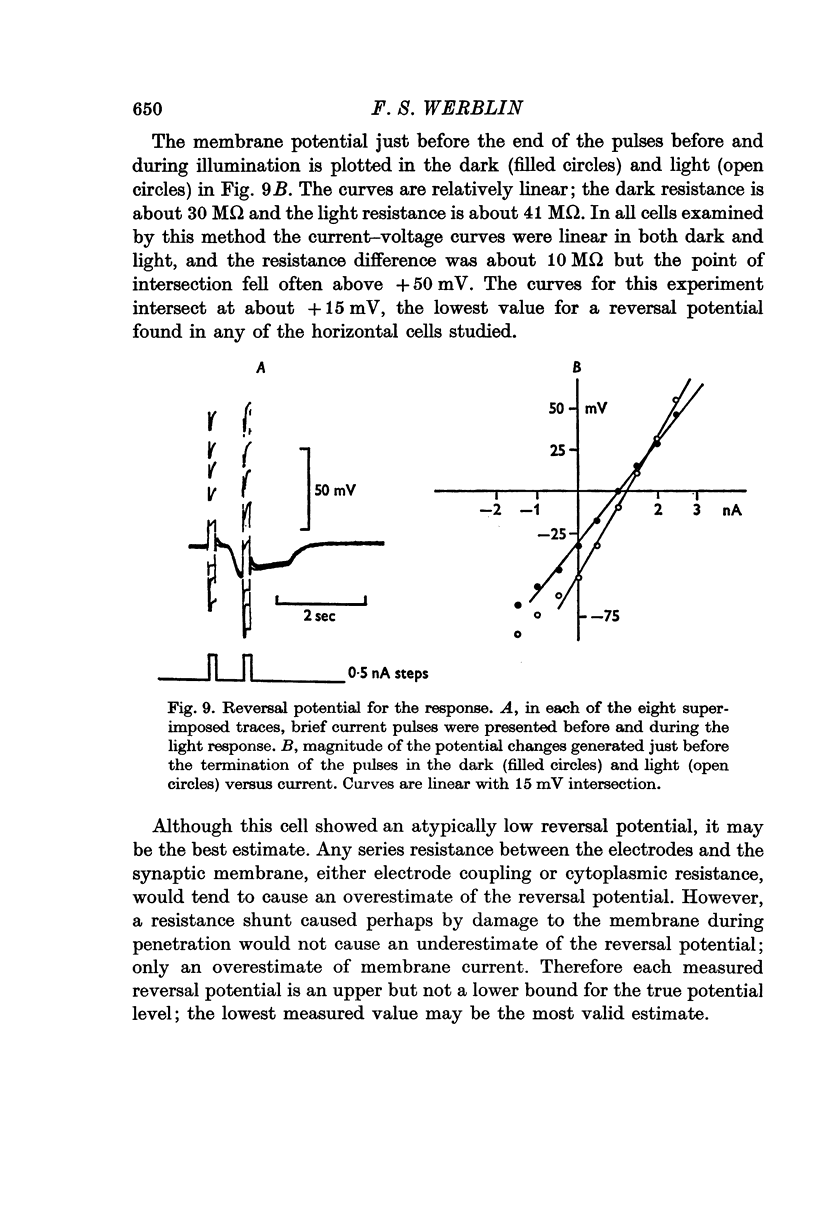

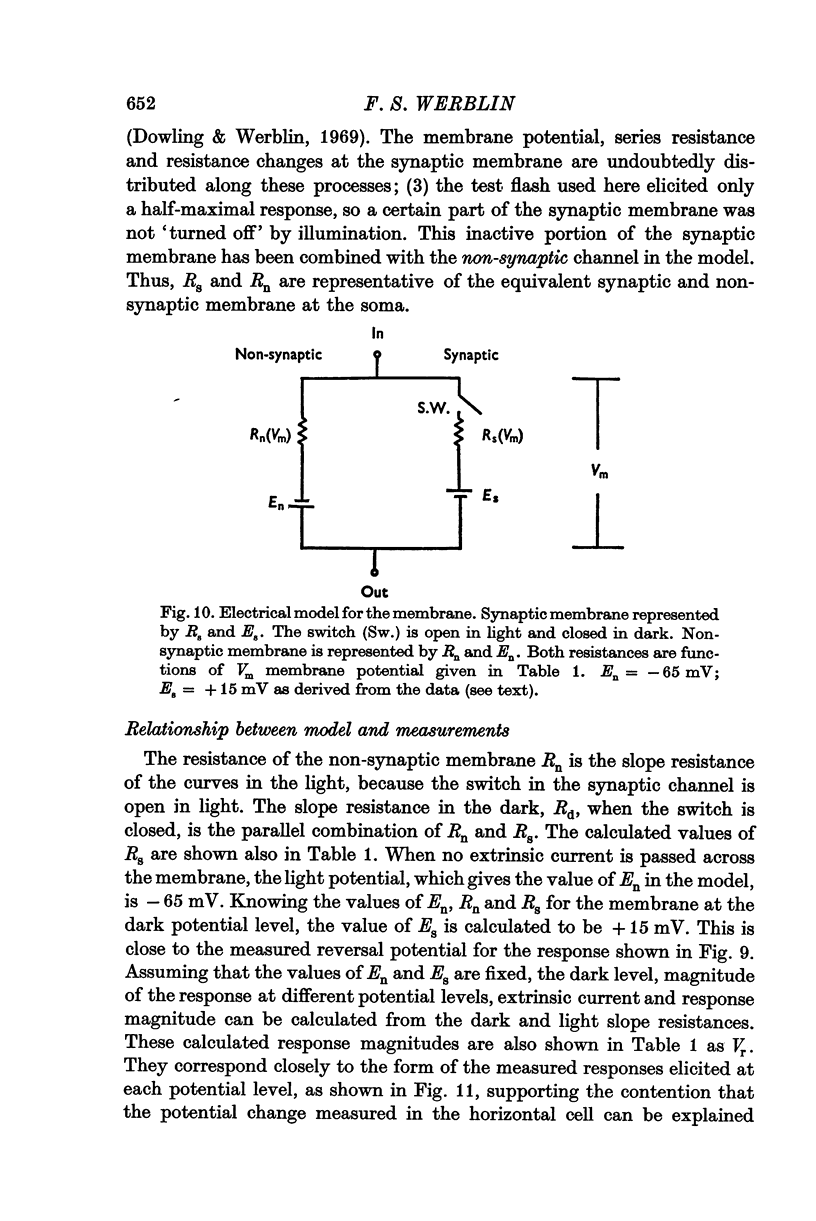
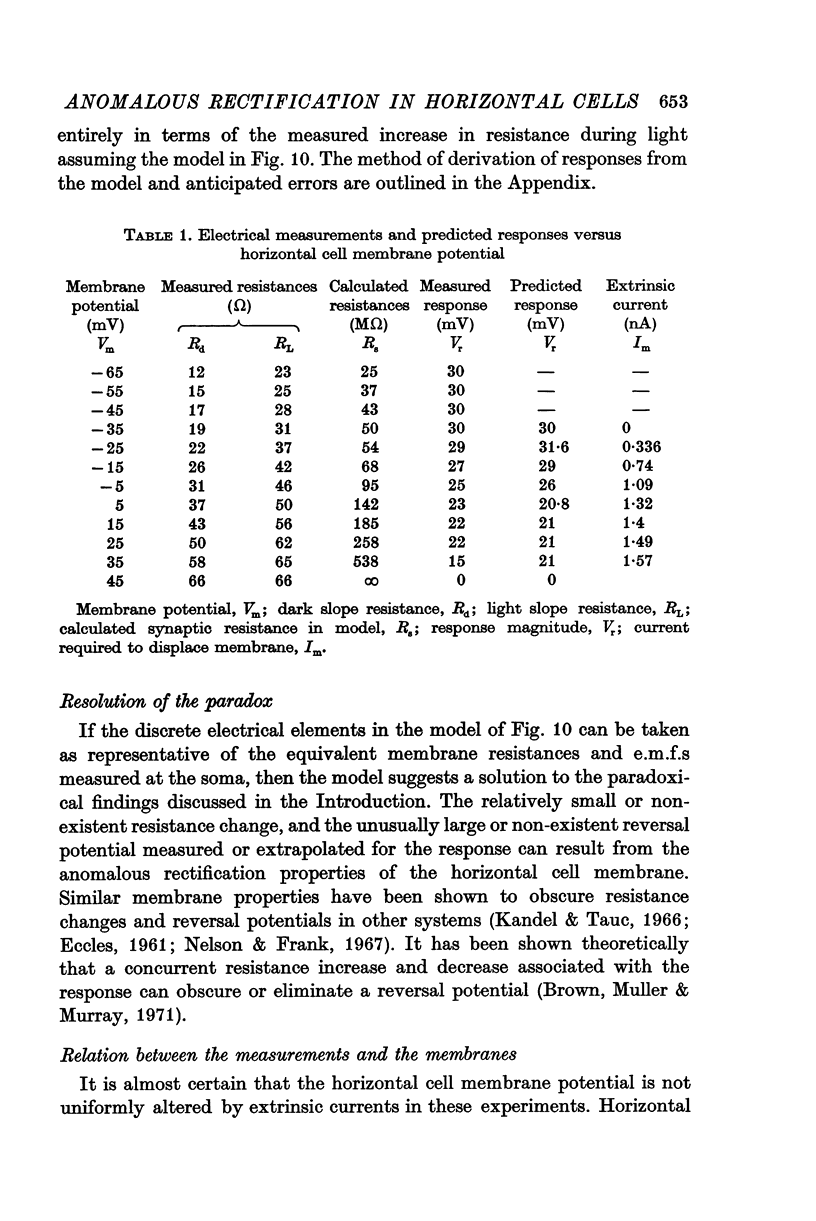
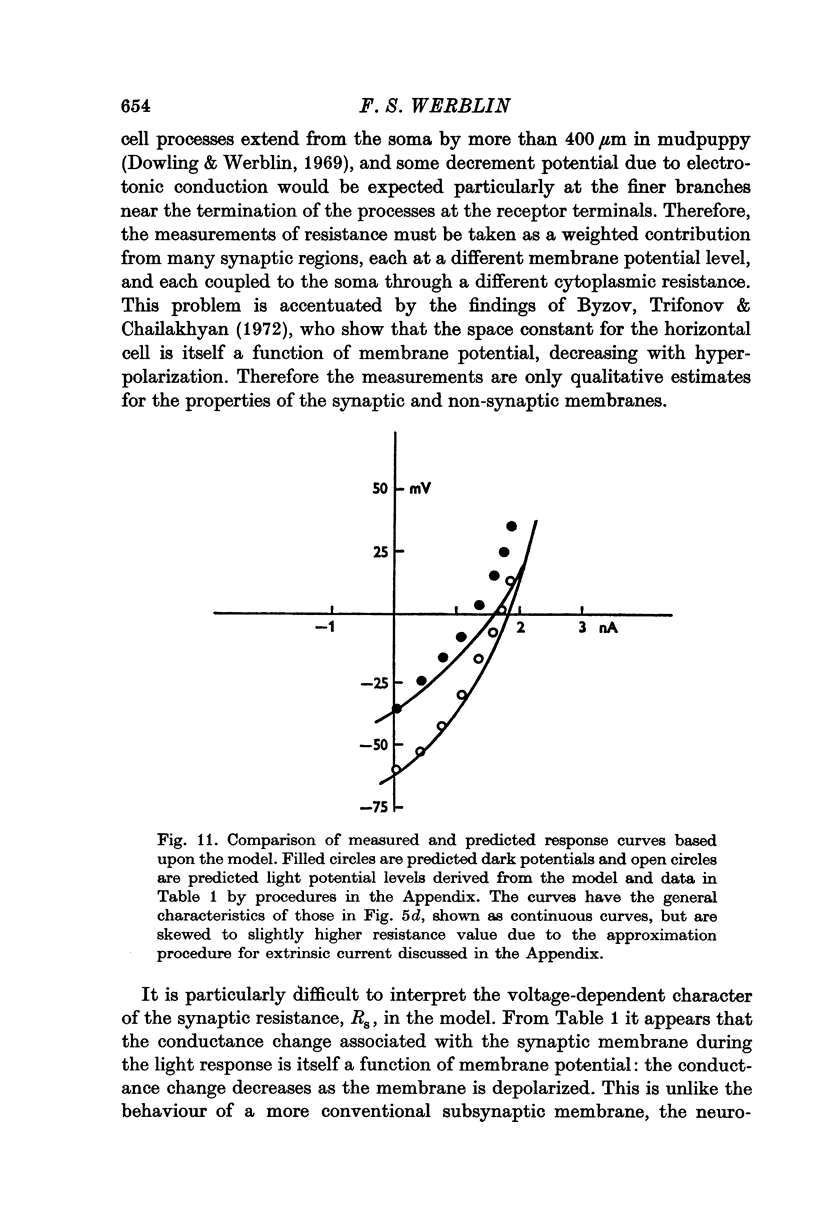



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Grundfest H. Analysis of depolarizing and hyperpolarizing inactivation responses in gymnotid electroplaques. J Gen Physiol. 1966 Sep;50(1):141–169. doi: 10.1085/jgp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Muller K. J., Murray G. Reversal potential for an electrophysiological event generated by conductance changes: mathematical analysis. Science. 1971 Oct 15;174(4006):318–318. doi: 10.1126/science.174.4006.318. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov Iu A., Chailakhian L. M. Vliianie poliarizatsii gorizontal'nykh kletok setchatki shchuki na rasprostranenie v nikh élektricheskikh potentsialov. Neirofiziologiia. 1972 Jan-Feb;4(1):90–96. [PubMed] [Google Scholar]
- Byzov A. L., Trifonov Iu A. Elektricheskie svoistva subsinapticheskoi i nesinapticheskoi membran gorizontal'nykh kletok setchatki cherepakhi. Neirofiziologiia. 1973 Jul-Aug;5(4):423–431. [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973 Mar 9;242(5393):101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C. The mechanism of synaptic transmission. Ergeb Physiol. 1961;51:299–430. [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOURAS P. Graded potentials of bream retina. J Physiol. 1960 Jul;152:487–505. doi: 10.1113/jphysiol.1960.sp006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Romey G. Responses anomales a des courants sous-liminaires de certaines membranes somatiques (neurones géants d'Helix pomatia). Analyse par la methode du voltage impose. Pflugers Arch. 1971;327(2):105–131. doi: 10.1007/BF00587365. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Cell junctions at the outer synaptic layer of the retina. Invest Ophthalmol. 1972 May;11(5):265–275. [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Maksimova E. M., Maksimov V. V. Ob izmenenii vkhodnogo soprotivleniia postoiannomu toku gorizontal'nykh kletok setchatki ryb pri vozbuzhdenii. Neirofiziologiia. 1971 Mar-Apr;3(2):210–216. [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A. An intracellular coaxial microelectrode--its construction and application. Med Electron Biol Eng. 1965 Oct;3(4):367–376. doi: 10.1007/BF02476131. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Trifonov Iu A., Chailakhian L. M., Byzov A. L. Issledovanie prirody élektricheskikh otvetov gorizontal'nykh kletok setchatki ryb. Neirofiziologiia. 1971 Jan-Feb;3(1):89–98. [PubMed] [Google Scholar]
- Trifonov Iu A., Utina I. A. Issledovanie mekhanizma deistviia toka na kletki L-tipa setchatki. Biofizika. 1966;11(4):646–652. [PubMed] [Google Scholar]
- WATANABE K., TOSAKA T., YOKOTA T. Effects of extrinsic electric current on the cyprinid fish EIRG (S-potential). Jpn J Physiol. 1960 Apr 29;10:132–141. doi: 10.2170/jjphysiol.10.132. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]


