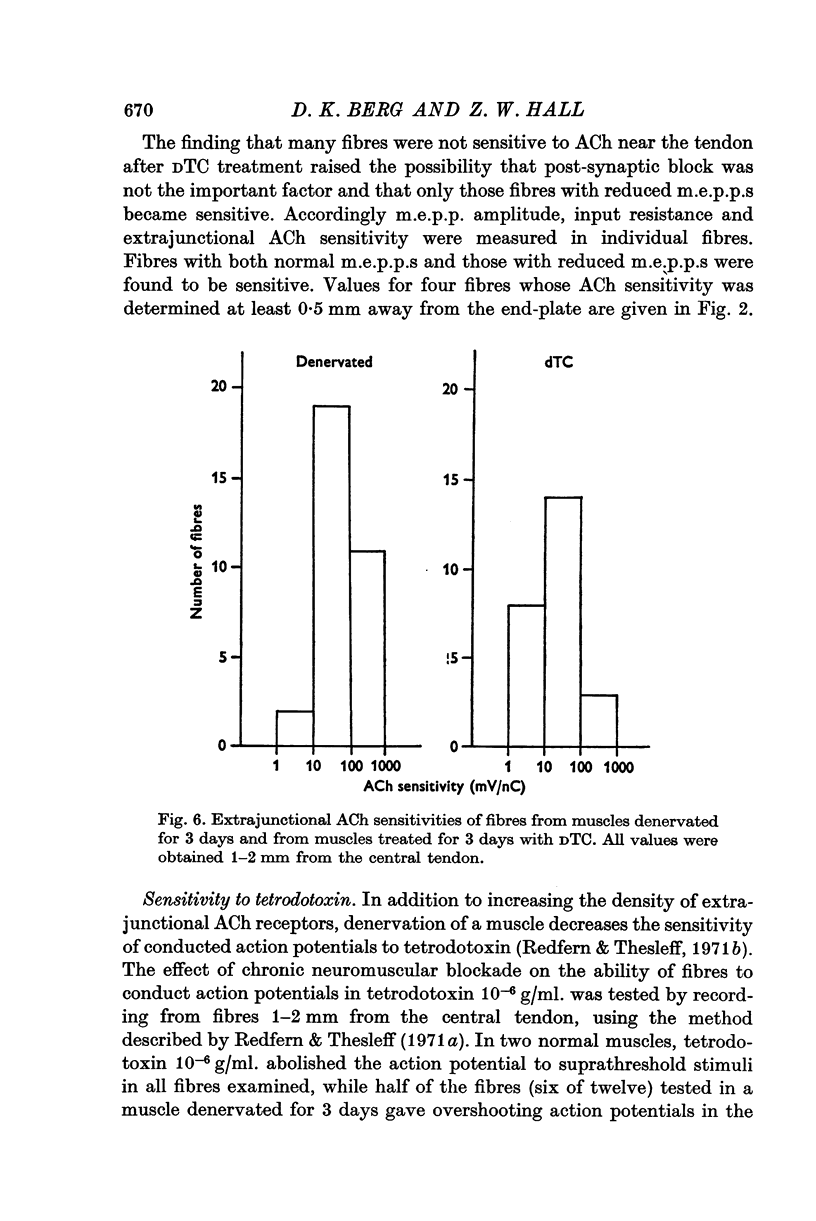
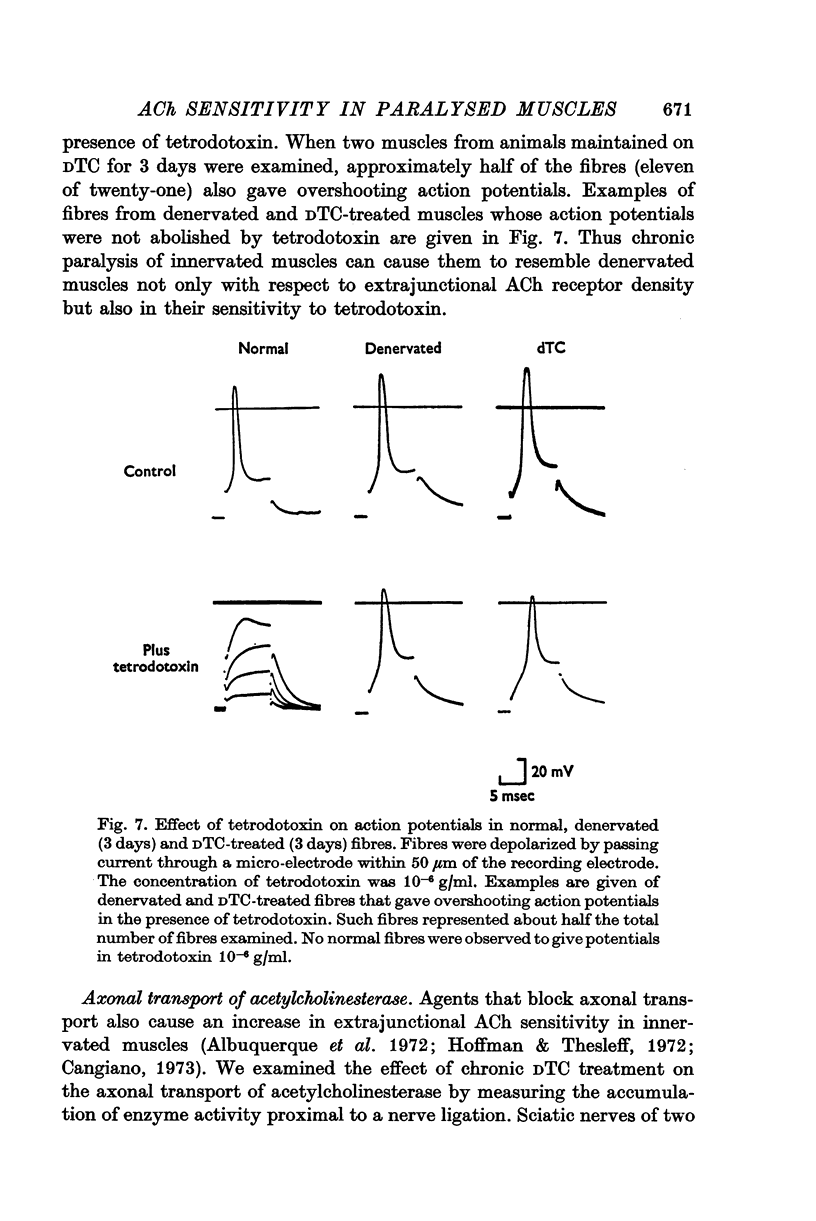
Abstract
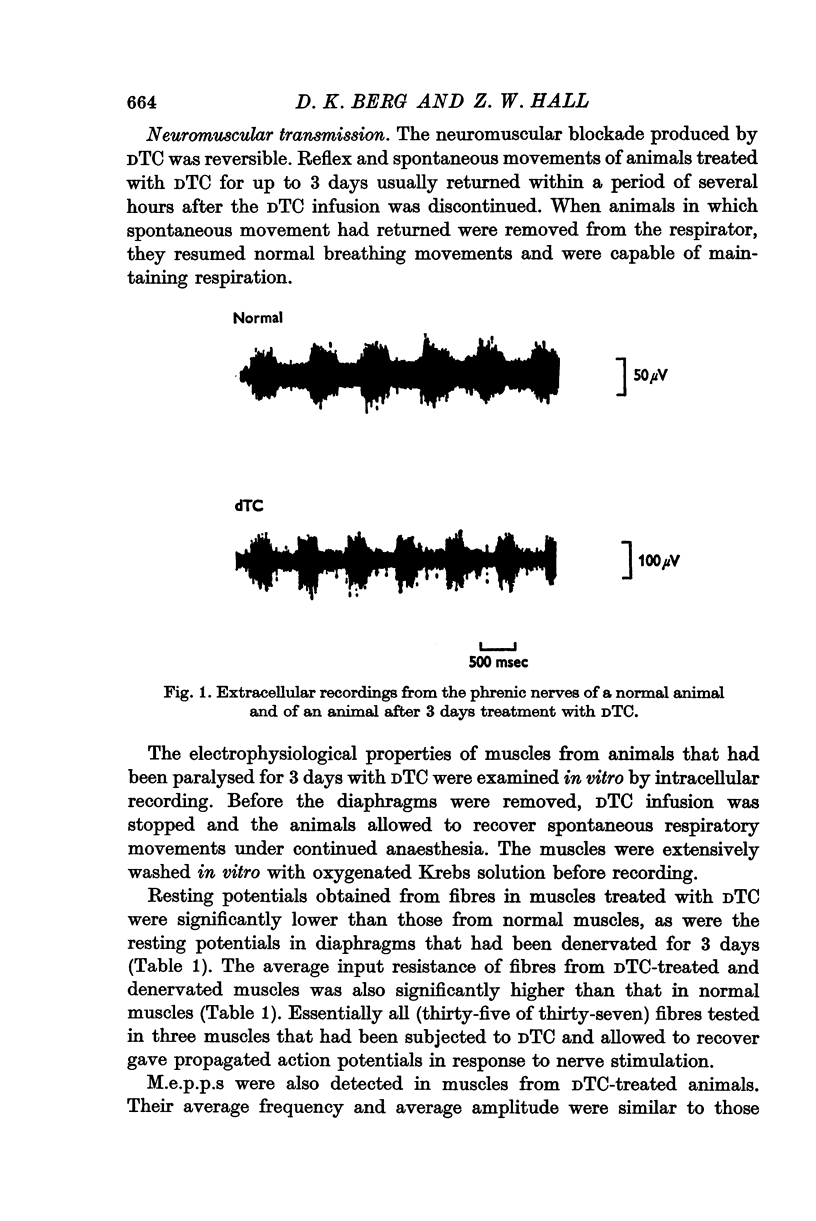
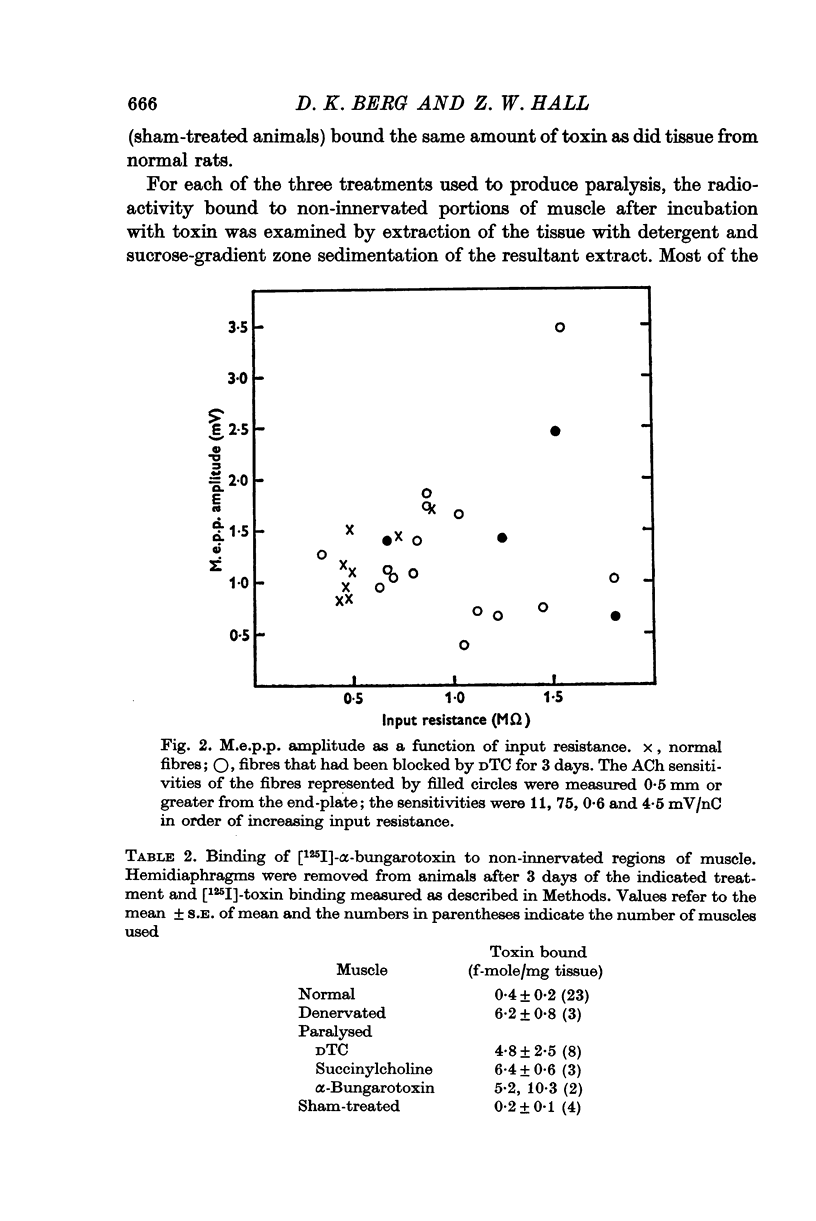
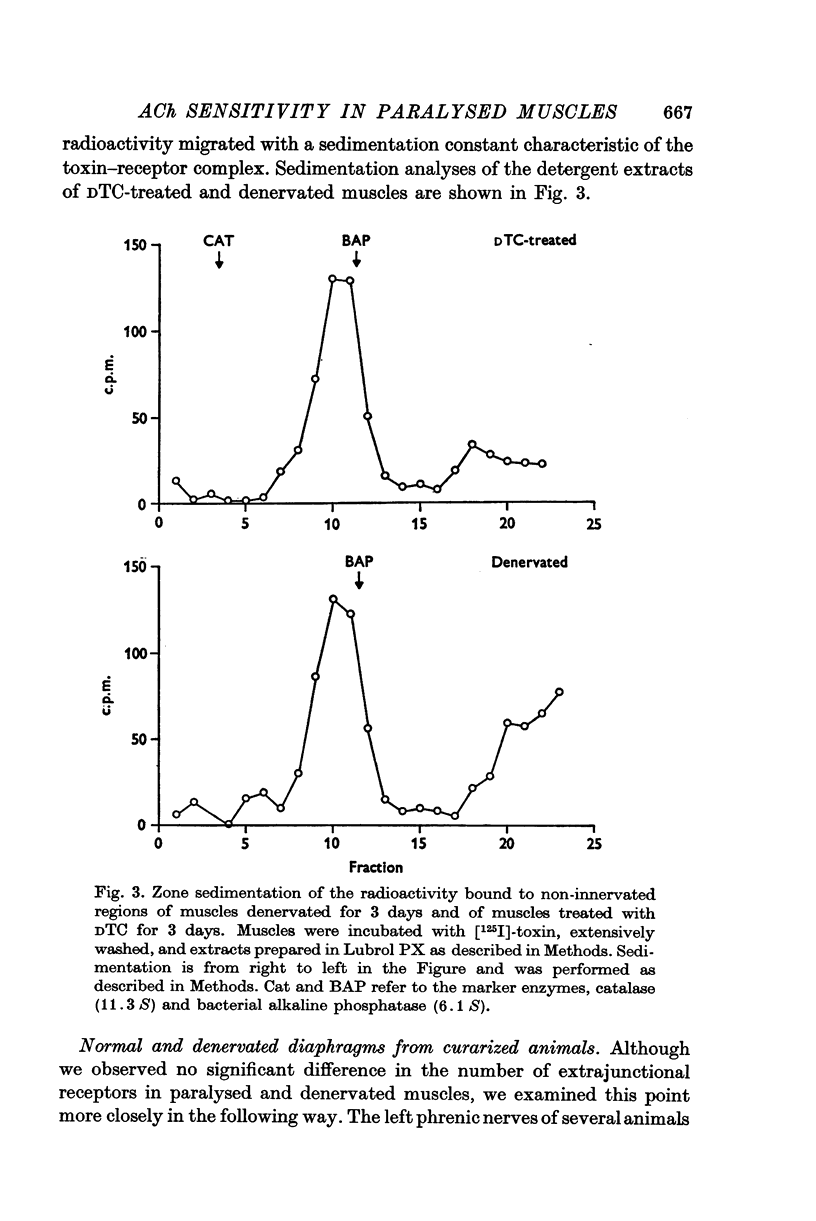
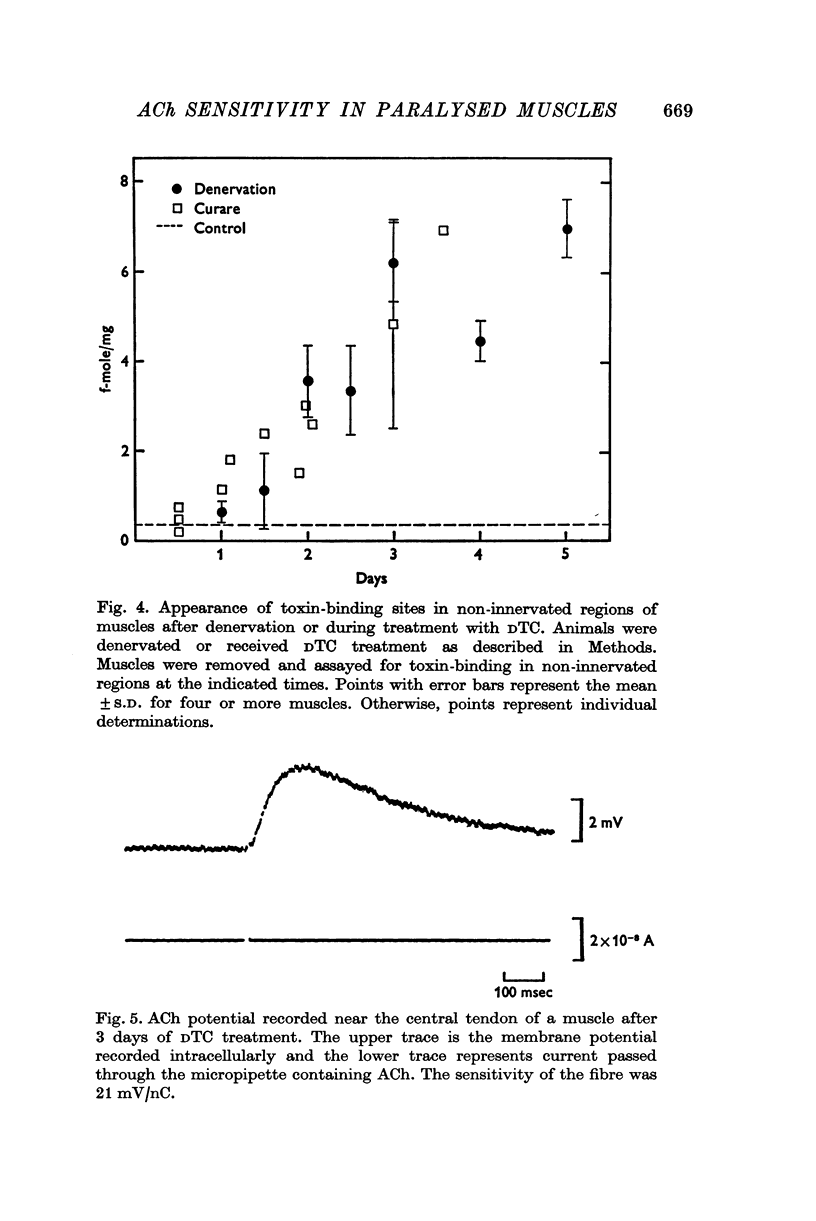
1. Anaesthetized rats were paralysed for periods of up to 3 days by chronic administration of D-tubocurarine (DTC), succinylcholine or alpha-bungarotoxin. 2. After 3 days of treatment with DTC, the phrenic nerve remained active. Neuromuscular transmission and spontaneous miniature end-plate potentials (m.e.p.p.s) were restored after removal of the DTC. Resting potentials and input resistances of muscle fibres that had been paralysed for 3 days were similar to those in denervated fibers. 3. Chronic neuromuscular blockade increased the binding of [125-I]-alpha-bungarotoxin by extrajunctional regions of muscle. The time course of the increase was similar to that seen after denervation. Binding to muscles from animals that were anaesthetized and respirated, but not paralysed, was not increased. 4. Three days of paralysis increased the sensitivity of the extrajunctional muscle membrane to acetylcholine (ACh) applied by iontophoresis. 5. Approximately the same proportion of muscle fibres from muscles paralysed for 3 days gave overshooting action potentials in the presence of tetrodotoxin 10-minus 6 g/ml. as did fibres form muscles denervated for 3 days. 6. Chronic paralysis did not change the accumulation of acetylcholinesterase above a ligation in the sciatic nerve. 7. These results are consistent with the idea that extrajunctional ACh sensitivity is normally controlled by muscle activity.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEANI L., BIANCHI C., LEDDA F. THE EFFECT OF TUBOCURARINE ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. J Physiol. 1964 Nov;174:172–183. doi: 10.1113/jphysiol.1964.sp007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Fate of alpha-bungarotoxin bound to acetylcholine receptors of normal and denervated muscle. Science. 1974 Apr 26;184(4135):473–475. doi: 10.1126/science.184.4135.473. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J. M., Merry E. H. Influence of d-tubocurarine, decamethonium and succinylcholine on repetitively evoked end-plate potentials. J Pharmacol Exp Ther. 1969 Jun;167(2):334–343. [PubMed] [Google Scholar]
- Cangiano A. Acetylcholine supersensitivity: the role of neurotrophic factors. Brain Res. 1973 Aug 17;58(1):255–259. doi: 10.1016/0006-8993(73)90842-1. [DOI] [PubMed] [Google Scholar]
- Cangiano A., Fried J. A. Proceedings: Neurotrophic control of skeletal muscle of the rat. J Physiol. 1974 May;239(1):31P–33P. [PubMed] [Google Scholar]
- Chang C. C., Lee C. Y. Electrophysiological study of neuromuscular blocking action of cobra neurotoxin. Br J Pharmacol Chemother. 1966 Nov;28(2):172–181. doi: 10.1111/j.1476-5381.1966.tb01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Regulation of muscle acetylcholine sensitivity by muscle activity in cell culture. Science. 1973 Jul 6;181(4094):76–78. doi: 10.1126/science.181.4094.76. [DOI] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- ELERT B. T., COHEN E. N. A micro spectrophotometric method for the analysis of minute concentrations of d-tubocurarine chloride in plasma. Am J Med Technol. 1962 May-Jun;28:125–134. [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Effect of chronic disuse of rat soleus neuromuscular junctions on postsynaptic membrane. J Neurophysiol. 1971 Jul;34(4):562–569. doi: 10.1152/jn.1971.34.4.562. [DOI] [PubMed] [Google Scholar]
- Fletcher P., Forrester T. The measurement of acetylcholine released from mammalian skeletal muscle in the presence of curare. J Physiol. 1970 Dec;211(2 Suppl):39P+–39P+. [PubMed] [Google Scholar]
- Gergis S. D., Dretchen K. L., Sokoll M. D., Long J. P. The effect of neuromuscular blocking agents on acetylcholine release. Proc Soc Exp Biol Med. 1971 Nov;138(2):693–695. doi: 10.3181/00379727-138-35970. [DOI] [PubMed] [Google Scholar]
- HEBB C. O., KRNJEVIC K., SILVER A. ACETYLCHOLINE AND CHOLINE ACETYLTRANSFERASE IN THE DIAPHRAGM OF THE RAT. J Physiol. 1964 Jun;171:504–513. doi: 10.1113/jphysiol.1964.sp007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. W., Thesleff S. Studies on the trophic influence of nerve on skeletal muscle. Eur J Pharmacol. 1972 Dec;20(3):256–260. doi: 10.1016/0014-2999(72)90182-3. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS T. R., THESLEFF S. Effects of motor inactivation on the chemical sensitivity of skeletal muscle. Acta Physiol Scand. 1961 Feb-Mar;51:136–141. doi: 10.1111/j.1748-1716.1961.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. The release of acetylcholine in the isolated rat diaphragm. J Physiol. 1961 Feb;155:246–262. doi: 10.1113/jphysiol.1961.sp006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T. Neurotrophic control of colchicine effects on muscle? Nature. 1974 May 31;249(456):473–474. doi: 10.1038/249473a0. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranish N., Ochs S. Fast axoplasmic transport of acetylcholinesterase in mammalian nerve fibres. J Neurochem. 1972 Nov;19(11):2641–2649. doi: 10.1111/j.1471-4159.1972.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Riker W. F., Jr, Okamoto M. Pharmacology of motor nerve terminals. Annu Rev Pharmacol. 1969;9:173–208. doi: 10.1146/annurev.pa.09.040169.001133. [DOI] [PubMed] [Google Scholar]
- Robert E. D., Oester Y. T. Absence of supersensitivity to acetylcholine in innervated muscle subjected to a prolonged pharmacologic nerve block. J Pharmacol Exp Ther. 1970 Jul;174(1):133–140. [PubMed] [Google Scholar]
- STANDAERT F. G. THE ACTION OF D-TUBOCURARINE ON THE MOTOR NERVE TERMINAL. J Pharmacol Exp Ther. 1964 Feb;143:181–186. [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]


