Abstract
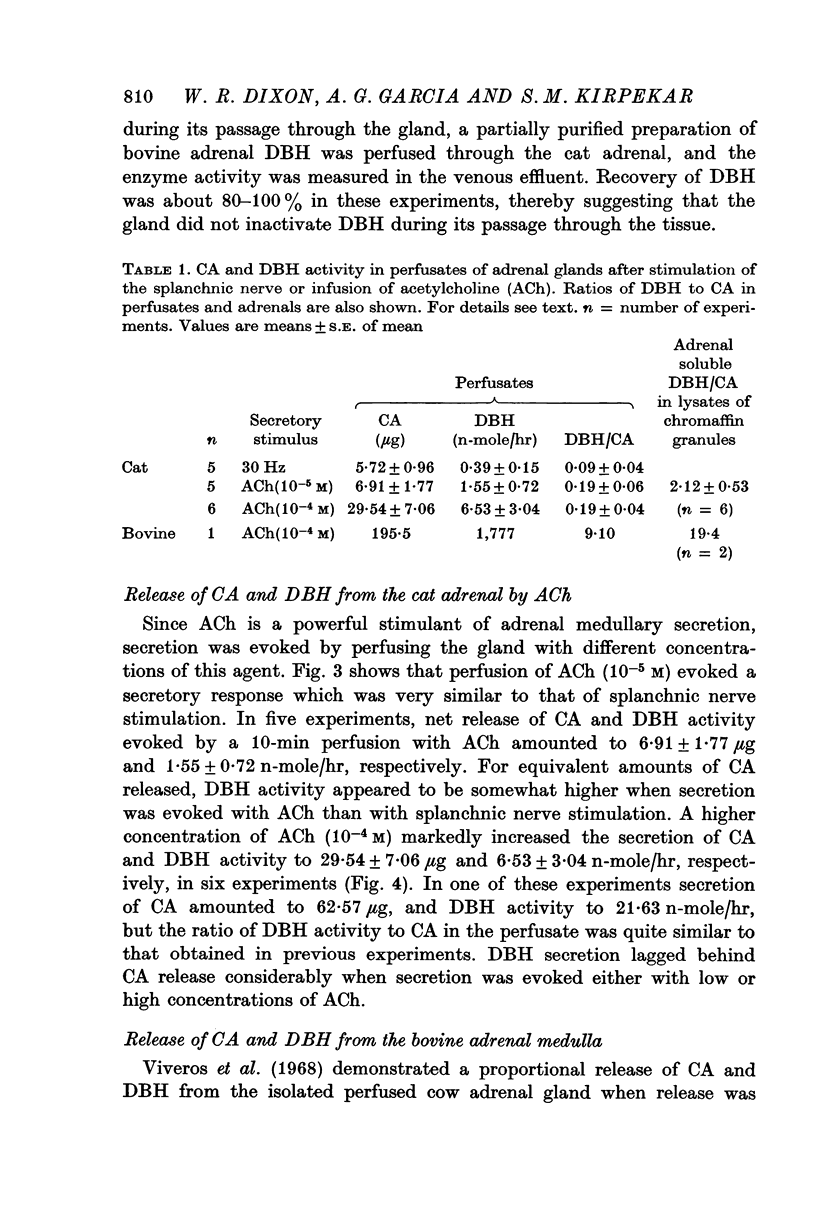
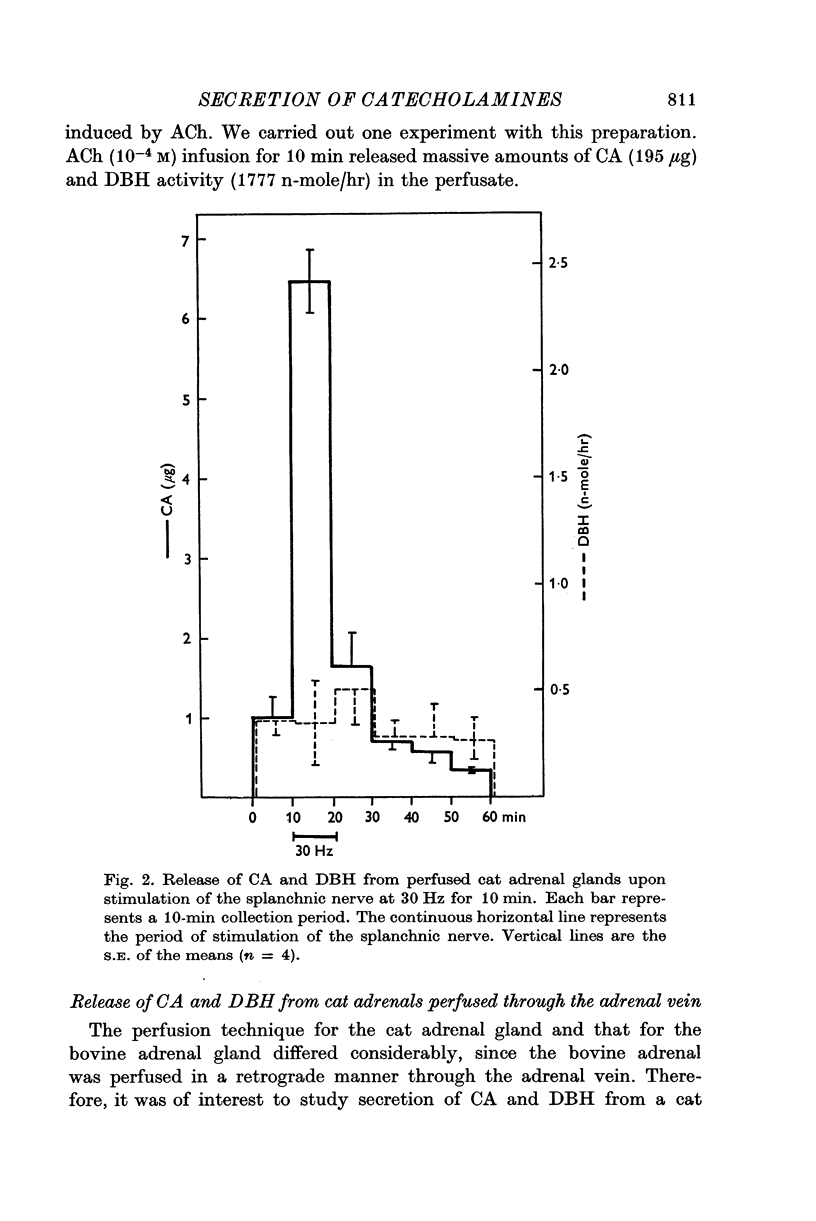
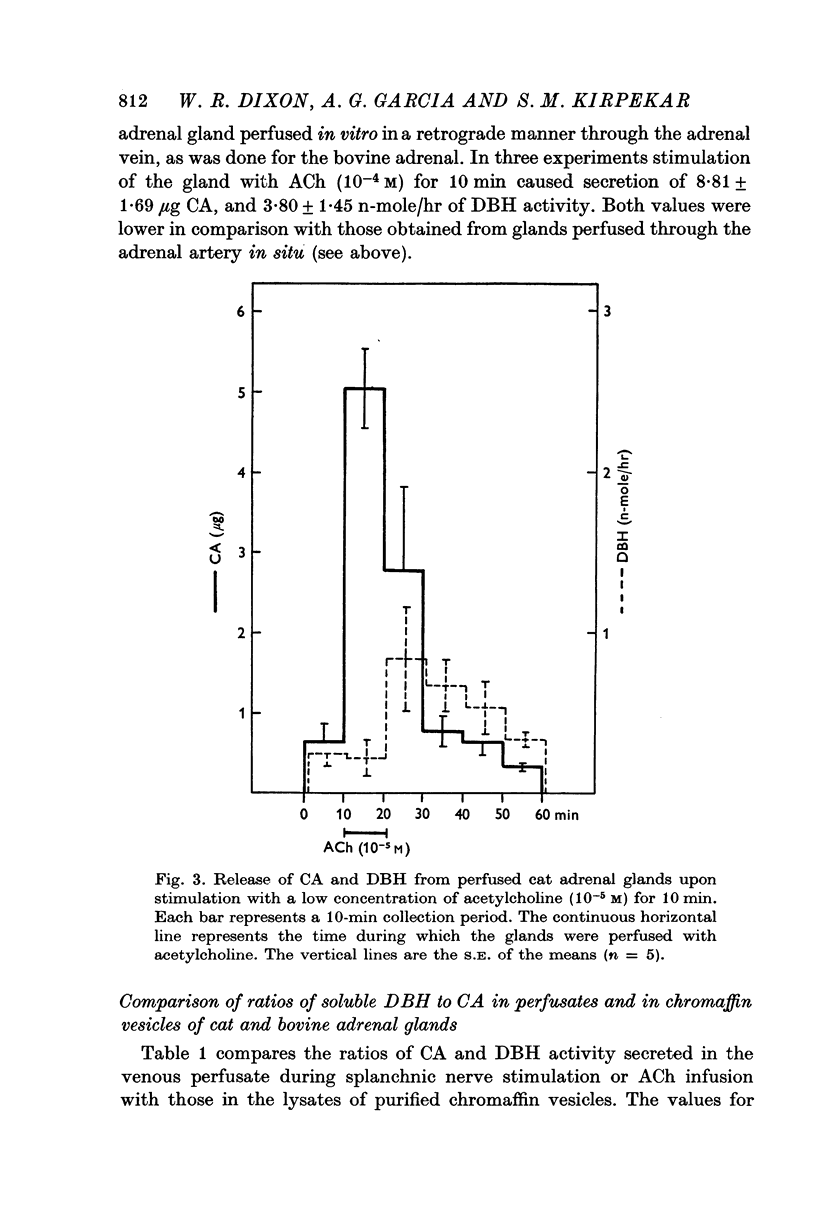
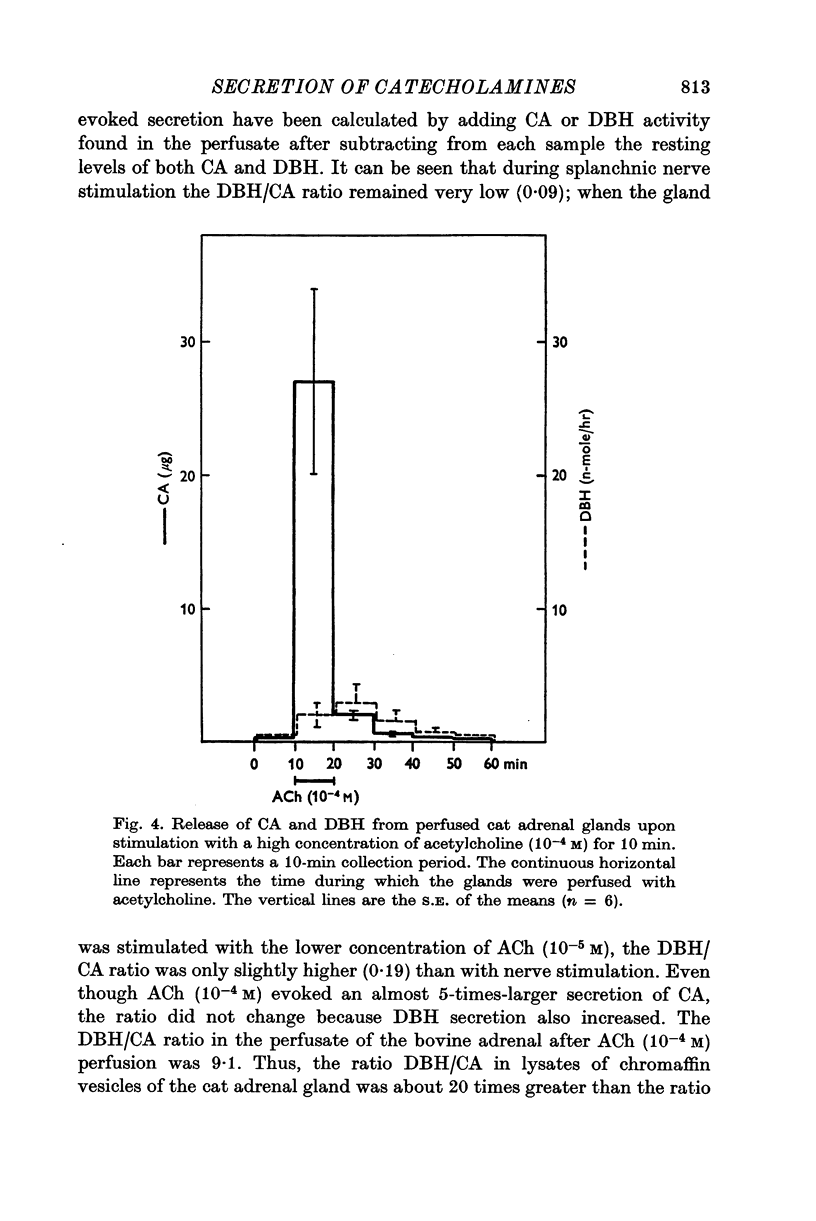
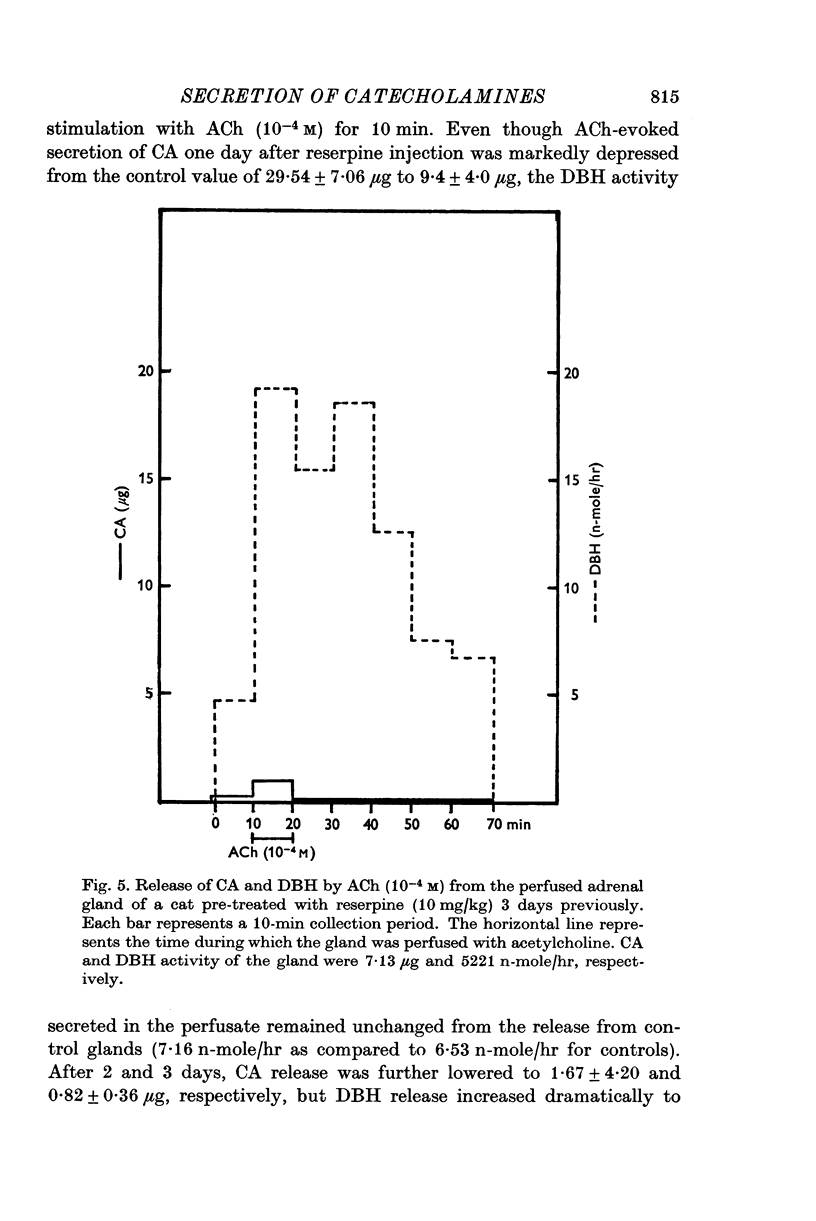
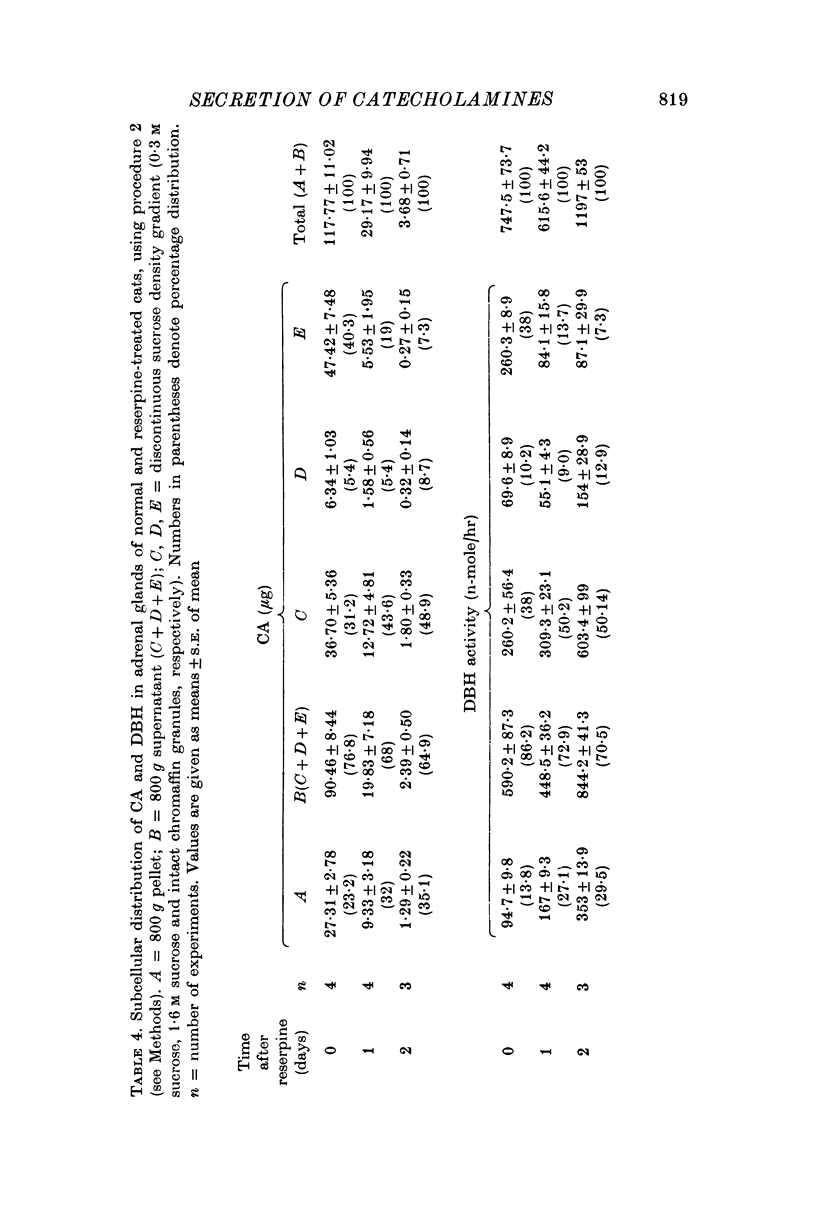
1. Secretion of catecholamines (CA) and dopamine beta-hydroxylase (DBH) activity from the perfused cat adrenal gland was studied following splanchnic nerve stimulation or infusion of acetylcholine (ACh). 2. Splanchnic nerve stimulation (30 Hz) or perfusion with a low concentration of ACh (10-minus5 M) caused a marked release of CA in the venous effluent, but release of DBH activity was minimal while a higher concentration of ACh (10-minus 4 M) enhanced the release of CA and DBH. 3. The ratio of DBH/CA released in the perfusate by splanchnic nerve stimulation or ACh infusion was only a small fraction of the ratio in the soluble lysate of purified chromaffin vesicles. 4. Following reserpine treatment, adrenal CA levels fell to 25% of the control value in 24 hr, remained depressed on days 2, 3, 4 and 5 at 5% of the control and recovered to 60% of the control value on the 6th day. DBH activity was unchanged from the control value at 24 hr after treatment, then rose as high as 5 times the control on the 5th day and was still twice the control value on the 6th day. 5. CA secretion in response to ACh (10-minus 4 M) perfusion was reduced to 30% of the control value on the first day after reserpine treatment, while DBH secretion was unchanged. On the 2nd day, CA secretion was depressed further to 5% of the control and remained at this low level up to 5 days after treatment while DBH secretion was twice the control value at 48 hr and then on days 3, 4 and 5 rose up to 5 times the control value. On the 6th day, secretion of CA recovered to 30% of the control while DBH secretion was now twice the control. 6. Isopycnic sucrose density (discontinuous) gradient centrifugation of vesicles from adrenal glands of control cats, and of cats given reserpine 1 or 2 days perviously, indicated that new vesicles or vesicles depleted of CA by reserpine had a lower equilibrium density than the original population of vesicles. 7. These results suggest that the release of CA is quantal in nature, but the release of DBH is not necessarily coupled with it. Release of DBH by ACh from reserpinized glands suggests that the vesicles which were once involved in secretion may be re-used for synthesis and storage of CA.
Full text
PDF




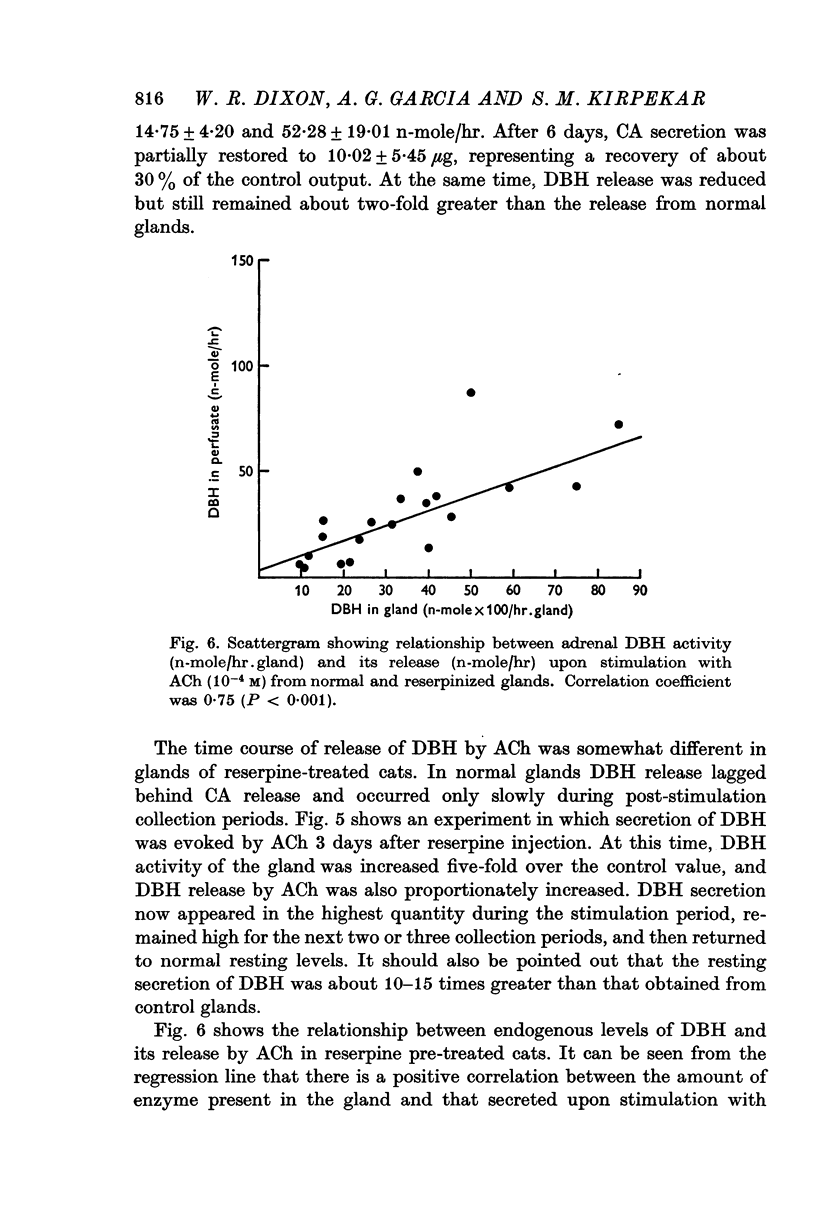






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
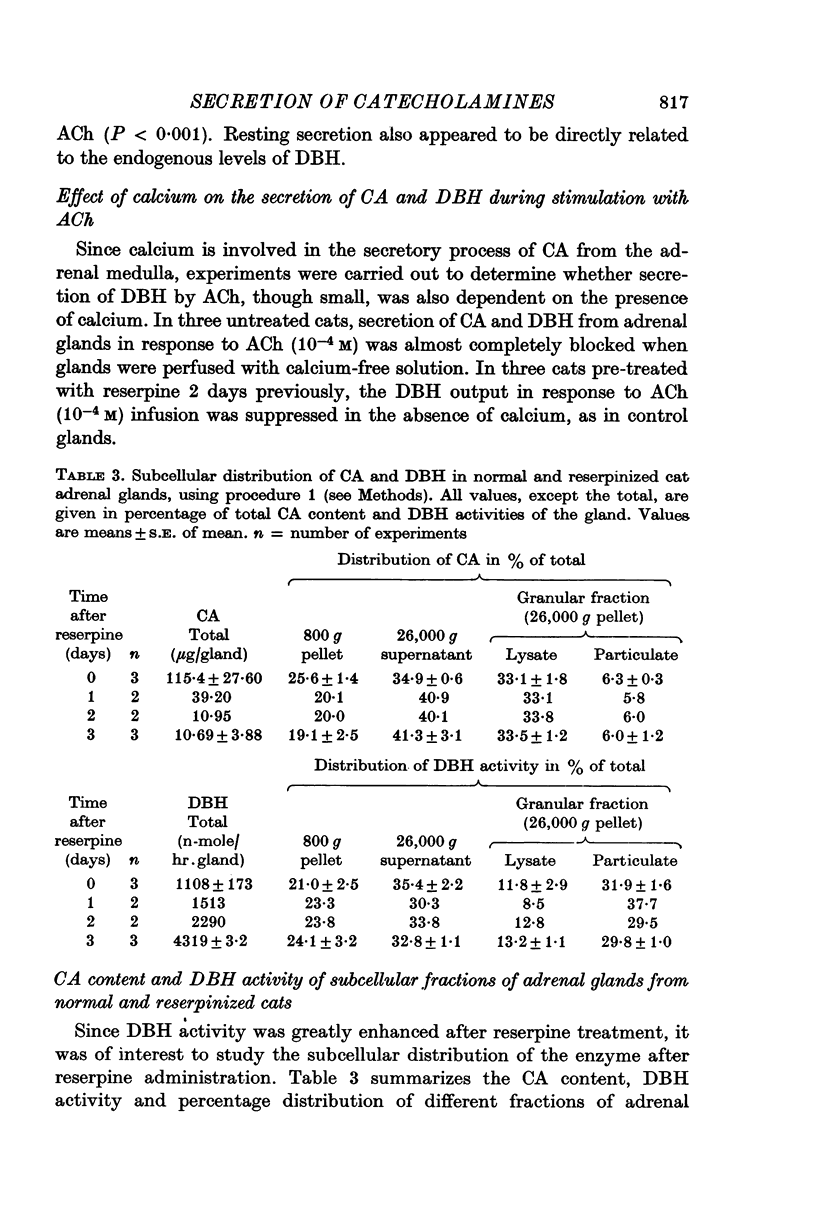
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Brimijoin S. Transport and turnover of dopamine- -hydroxylase (EC 1.14.2.1) in sympathetic nerves of the rat. J Neurochem. 1972 Sep;19(9):2183–2193. doi: 10.1111/j.1471-4159.1972.tb05127.x. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E., VAZ FERREIRA A. Electron microscope study of the excretion of cathecol-containing droplets in the adrenal medulla. Exp Cell Res. 1957 Jun;12(3):568–574. doi: 10.1016/0014-4827(57)90172-6. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolysed) during release of catecholamines. J Physiol. 1966 Mar;183(1):249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Bonnay M. An assay for dopamine-beta-hydroxylase activity in tissues and serum. Experientia. 1971 Jun;27(6):632–633. doi: 10.1007/BF02136929. [DOI] [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Poisner A. M., Douglas W. W. The fate of the chromaffin granule during catecholamine release from the adrenal medulla. I. Unchanged efflux of phospholipid and cholesterol. Biochem Pharmacol. 1967 Nov;16(11):2095–2100. doi: 10.1016/0006-2952(67)90006-8. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Connett R. J., Kirshner N. Mechanism of secretion from the adrenal medulla. 3. Studies of dopamine beta-hydroxylase as a marker for catecholamine storage vesicle membranes in rabbit adrenal glands. Mol Pharmacol. 1969 Jan;5(1):60–68. [PubMed] [Google Scholar]


