Abstract
1. Changes of extracellular K+ concentration, [K]e, arising in the spinal cord of the cat in response to an afferent stimulation were studied by means of K+-specific micro-electrodes.
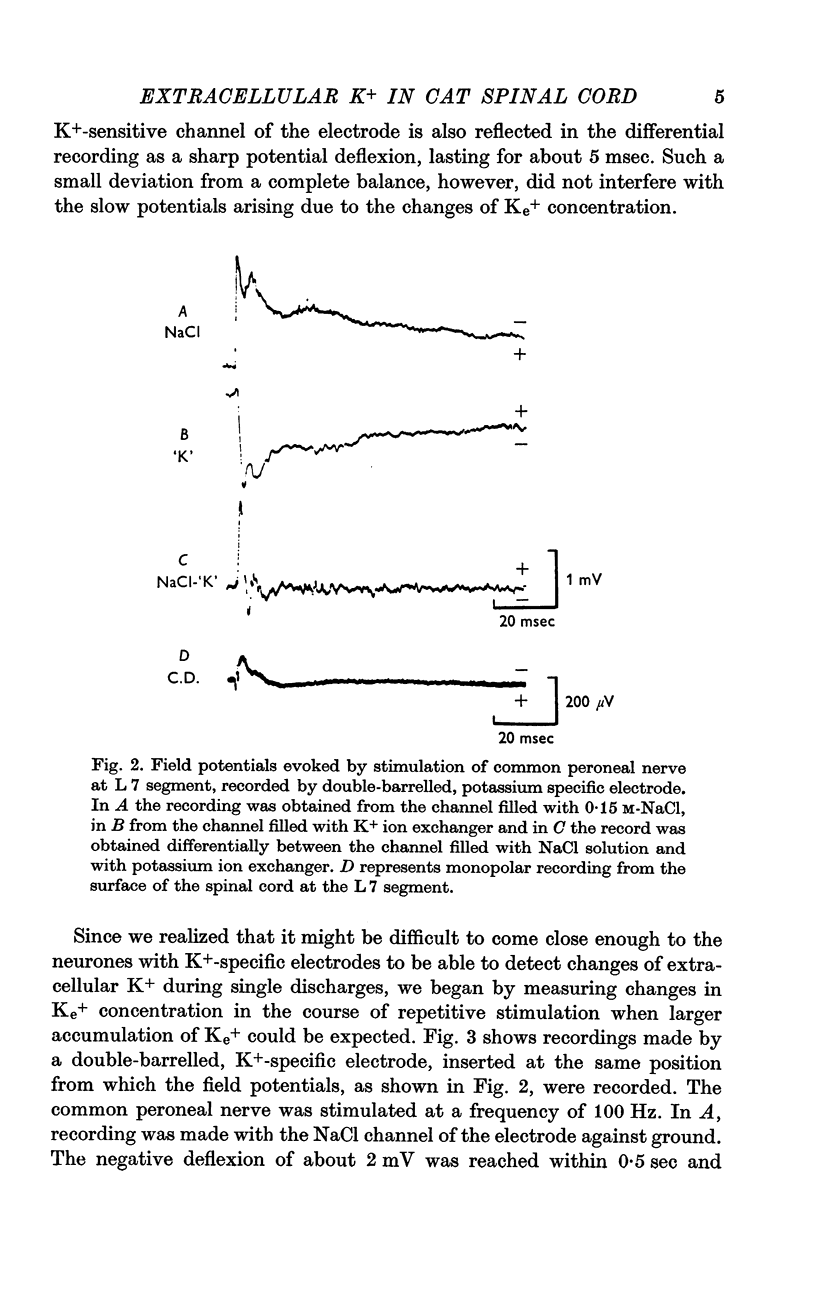
2. In the most active areas of the spinal cord a single volley in a large afferent input like the common peroneal nerve or the posterior tibial nerve produced a transient increase in [K]e of 0·05-0·1 mM, which reached its peak in 0·2-0·3 sec and it declined in about 3 sec.
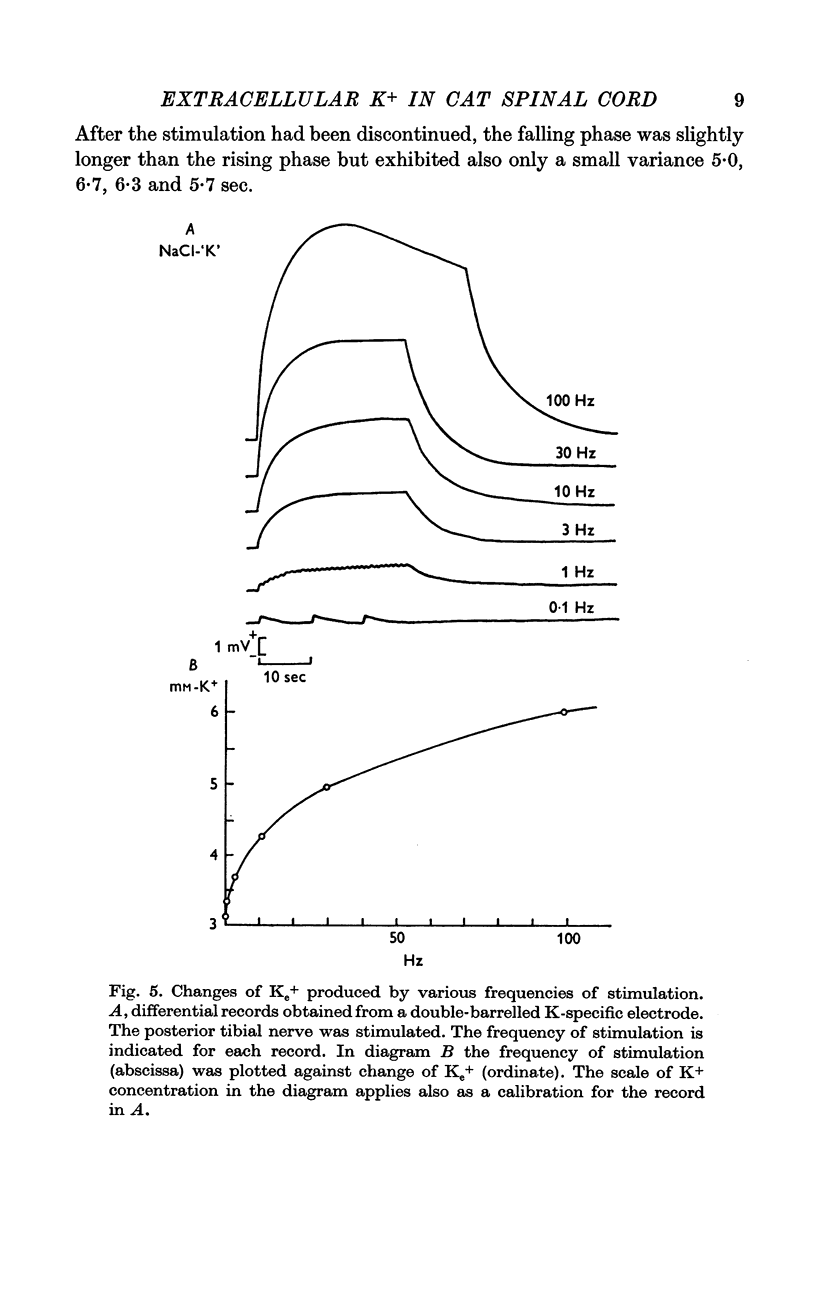
3. Much higher increases in [K]e were found during repetitive stimulation of an afferent input. The highest increase (by 3 mM) was at 100 Hz, but even at 1 Hz a significant increase of 0·25 mM was observed. Equilibration of accumulated K+ was slow with a time constant of about 6 sec, which is much longer than could be expected for the same process in free solution.
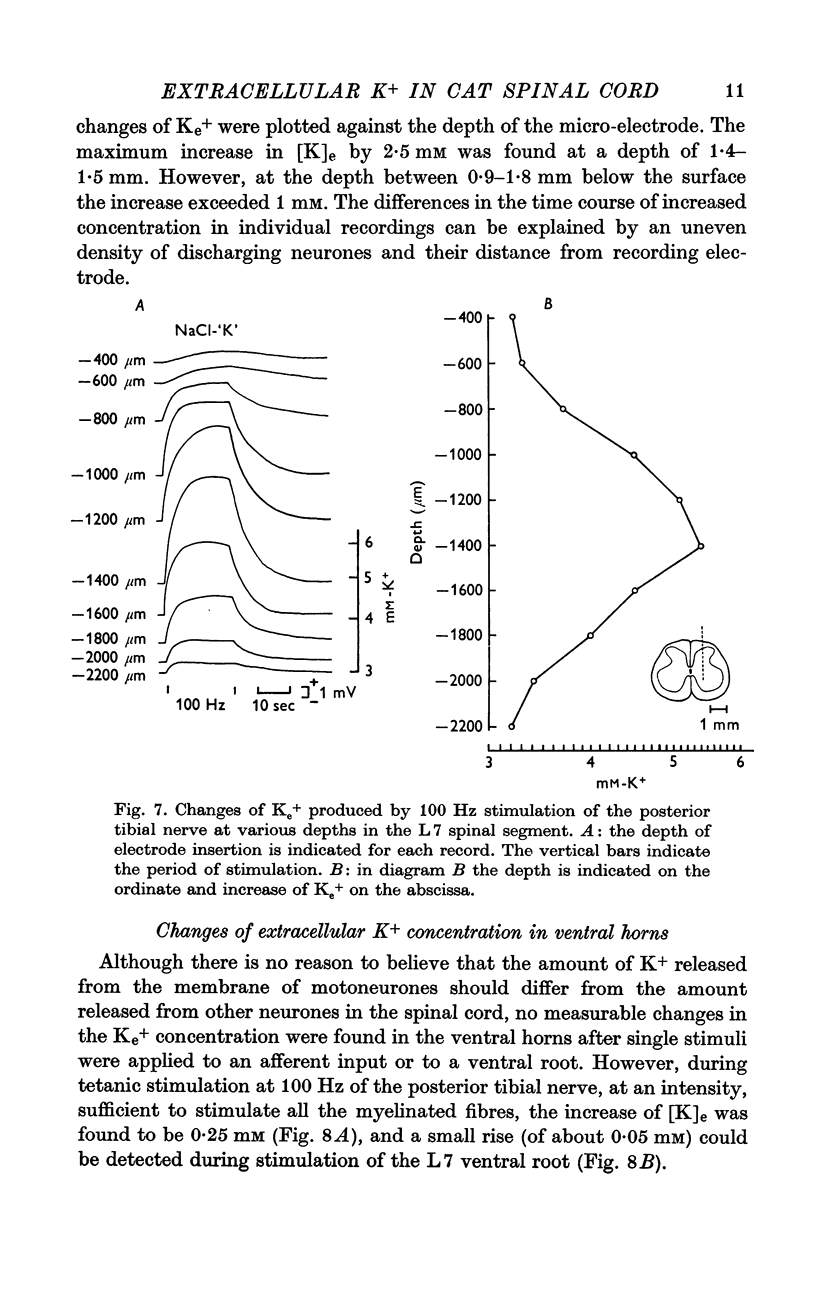
4. A characteristic distribution of increased [K]e was found in the spinal cord in response to 100 Hz afferent stimulation. The highest increase of 3 mM was found in and around the intermediate nucleus, but at depths between 0·9-1·8 mm the [K]e increase exceeded 1 mM.
5. In the ventral horns afferent stimulation (100 Hz) increased [K]e by 0·25 mM, while the same stimulation of the ventral root resulted in a [K]e increase of less than 0·05 mM.
6. The consequences of Ke+ accumulation after neuronal discharge are discussed in respect to its possible role in the depolarization of primary afferent terminals.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELFAN S., TARLOV I. M. ALTERED NEURON POPULATION IN L7 SEGMENT OF DOGS WITH EXPERIMENTAL HIND-LIMB RIGIDITY. Am J Physiol. 1963 Sep;205:606–616. doi: 10.1152/ajplegacy.1963.205.3.606. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. A morphological basis for pre-synaptic inhibition? Nature. 1962 Jan 6;193:82–83. doi: 10.1038/193082a0. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. Electron microscopy of presynaptic organelles of the spinal cord. J Anat. 1963 Jan;97:101–106. [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Reduction of transmitter output by depolarization. Nature. 1962 Mar 31;193:1294–1295. doi: 10.1038/1931294a0. [DOI] [PubMed] [Google Scholar]
- Hnik P., Vyskocil F., Kriz N., Holas M. Work-induced increase of extracellular potassium concentration in muscle measured by ion-specific electrodes. Brain Res. 1972 May 26;40(2):559–562. doi: 10.1016/0006-8993(72)90162-x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Extracellular K + activity and slow potential changes in spinal cord and medulla. Can J Physiol Pharmacol. 1972 Dec;50(12):1214–1217. doi: 10.1139/y72-177. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the spinal cord. UCLA Forum Med Sci. 1969;11:231–265. [PubMed] [Google Scholar]
- Lux H. D., Neher E. The equilibration time course of (K + ) 0 in cat cortex. Exp Brain Res. 1973 Apr 30;17(2):190–205. doi: 10.1007/BF00235028. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Prince D. A., Lux H. D., Neher E. Measurement of extracellular potassium activity in cat cortex. Brain Res. 1973 Feb 28;50(2):489–495. doi: 10.1016/0006-8993(73)90758-0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujec E., Beránek R. Differential high-impedance DC amplifier with negative input capacity. Physiol Bohemoslov. 1967;16(1):89–96. [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]


