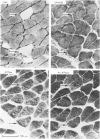
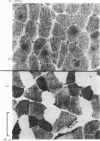
Abstract
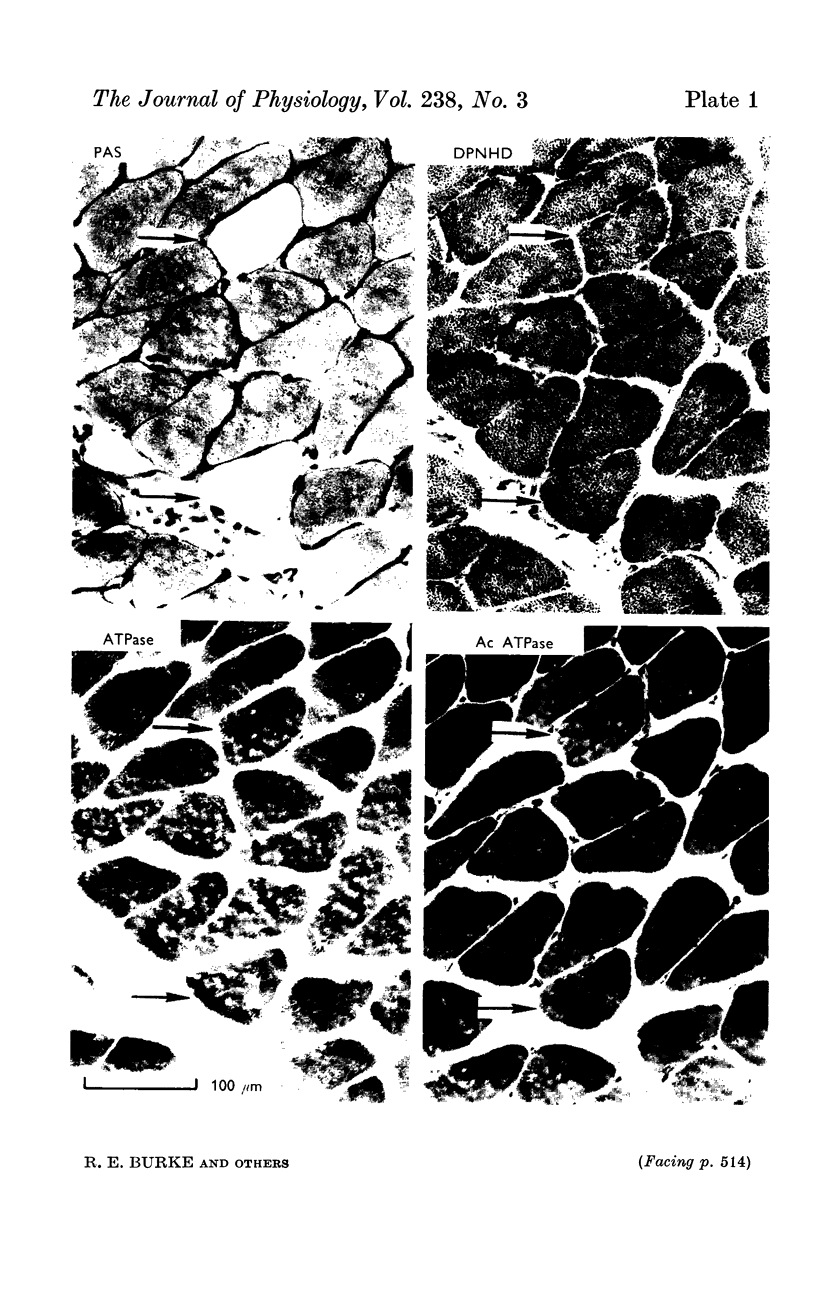
1. Physiological properties of motor units in the soleus muscle were studied in anaesthetized cats using intracellular stimulation of motoneurones to ensure functional isolation of single units. The muscle fibres belonging to 6 units were identified by glycogen depletion following prolonged stimulation, permitting analysis of their histochemical profiles and anatomical organization.
2. The studied units in soleus were all classed as type S and were extremely resistant to fatigue during prolonged stimulation. Twitch contraction times ranged from 64 to 131 msec (mean 97·1 msec) and tetanic tensions ranged from 3·5 to 36 g (mean 10·5 g). Most units exhibited depression of twitch tension in the wake of a short high-frequency tetanus and few of the units showed any significant degree of post-tetanic twitch potentiation.
3. Muscle fibres belonging to single soleus motor units were found to be scattered through territorial volumes occupying a large fraction of the total muscle volume. The available data suggest that different soleus motoneurones may innervate from less than 50 to more than 400 muscle fibres, with an average innervation ratio between 140 and 190 muscle fibres per unit.
4. The results were compared with observations on type S motor units in the synergist gastrocnemius, obtained under similar conditions. The evidence suggests that soleus units are not equivalent to the type S units of the mixed gastrocnemius but rather constitute a unique population.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- BOWMAN W. C., GOLDBERG A. A., RAPER C. A comparison between the effects of a tetanus and the effects of sympathomimetic amines on fast- and slow-contracting mammalian muscles. Br J Pharmacol Chemother. 1962 Dec;19:464–484. doi: 10.1111/j.1476-5381.1962.tb01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., von Euler U. S. The after effects of a tetanus on mammalian muscle. J Physiol. 1938 Jun 14;93(1):39–60. doi: 10.1113/jphysiol.1938.sp003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Jankowska E., ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol. 1970 May;207(3):709–732. doi: 10.1113/jphysiol.1970.sp009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F. On the localization of sarcotubular ATPase activity in mammaliam skeletal muscle. Histochemie. 1967;11(2):97–111. doi: 10.1007/BF00571715. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Erroneous interpretations which may result from application of the "myofibrillar ATPase" histochemical procedure to developing muscle. Exp Neurol. 1972 Mar;34(3):465–475. doi: 10.1016/0014-4886(72)90042-8. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Guth L., Yellin H. The dynamic nature of the so-called "fiber types" of nammalian skeletal muscle. Exp Neurol. 1971 May;31(2):227–300. doi: 10.1016/0014-4886(71)90196-8. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- Romanul F. C., Van der Meulen J. P. Slow and fast muscles after cross innervation. Enzymatic and physiological changes. Arch Neurol. 1967 Oct;17(4):387–402. doi: 10.1001/archneur.1967.00470280053006. [DOI] [PubMed] [Google Scholar]
- STANDAERT F. G. THE MECHANISMS OF POST-TETANIC POTENTIATION IN CAT SOLEUS AND GASTROCNEMIUS MUSCLES. J Gen Physiol. 1964 May;47:987–1001. doi: 10.1085/jgp.47.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Hanzlíková V., Pierobon S. Relations between structure and function in rat skeletal muscle fibers. J Cell Biol. 1970 Oct;47(1):107–119. doi: 10.1083/jcb.47.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]