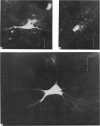
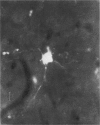
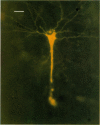
Abstract
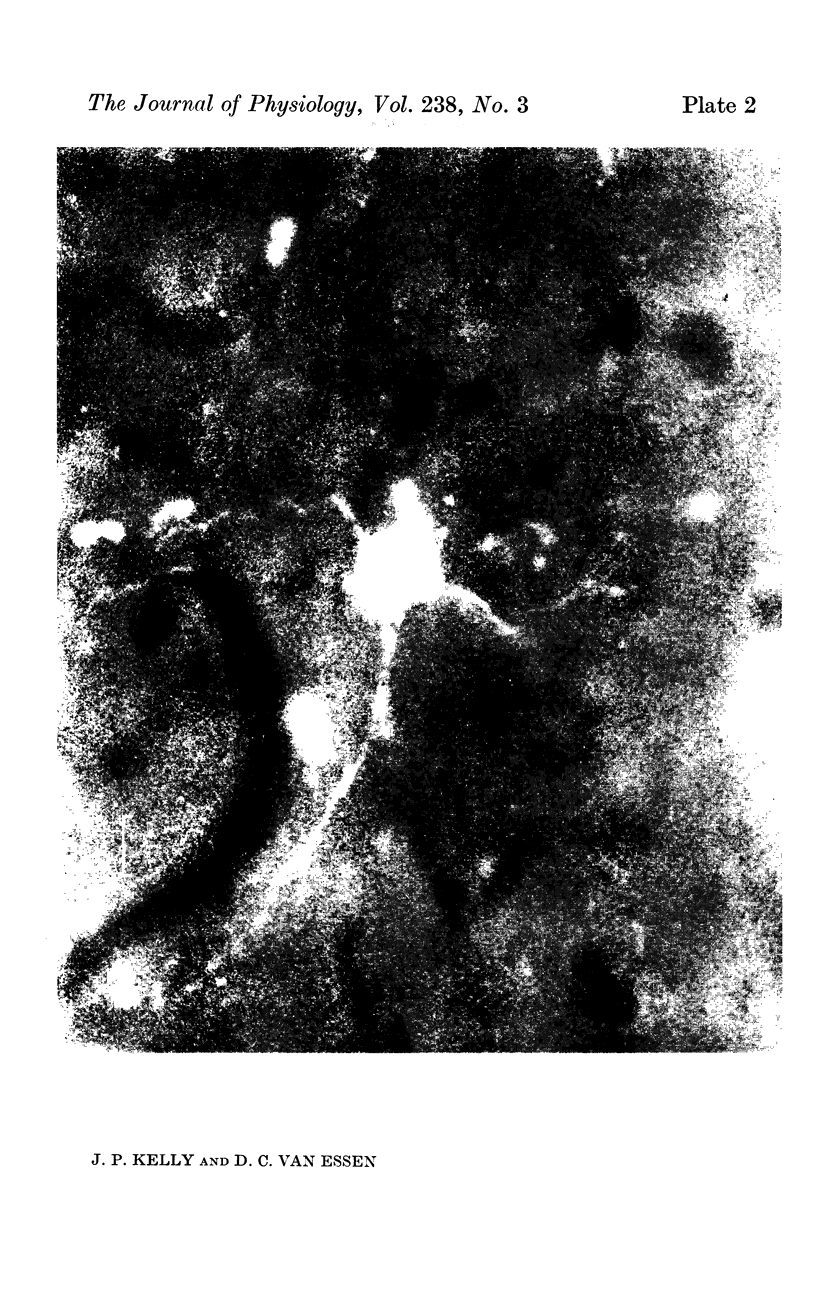
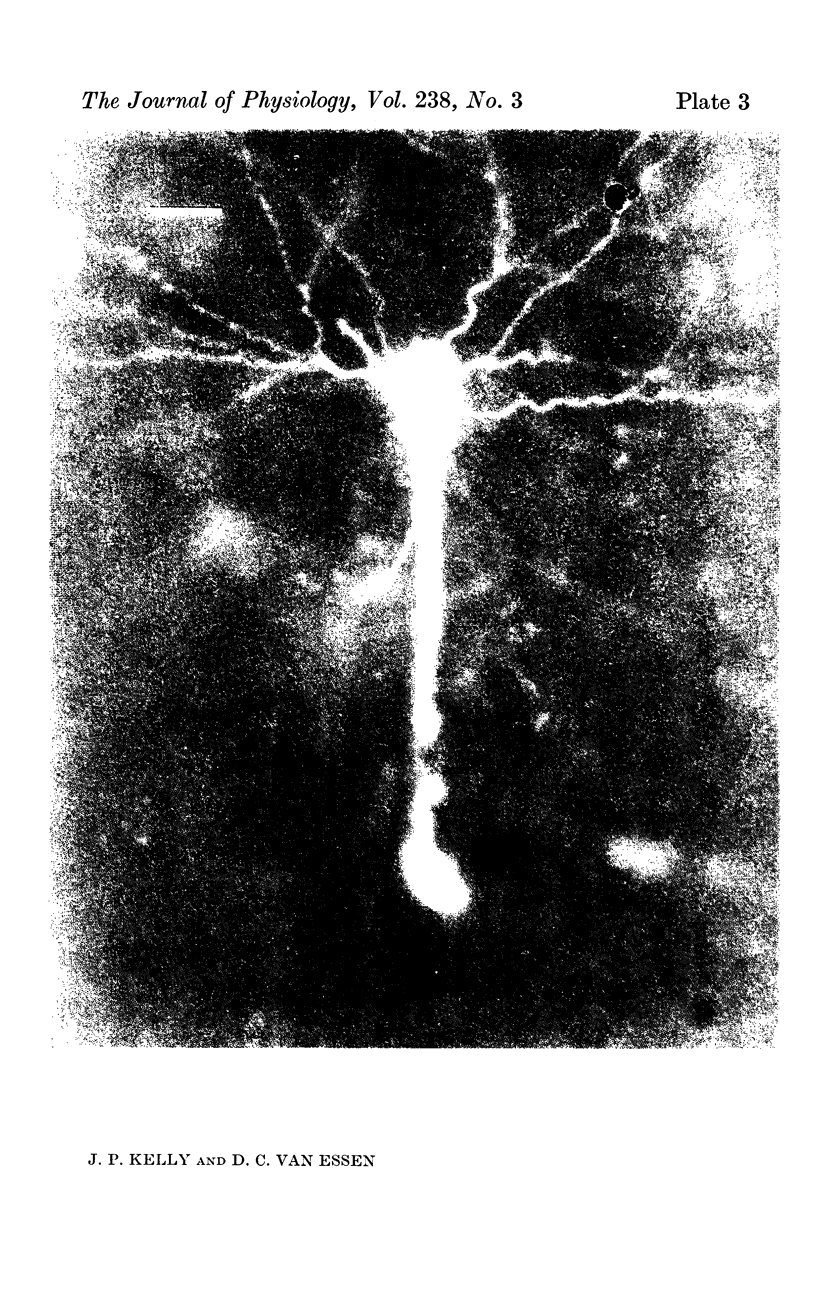
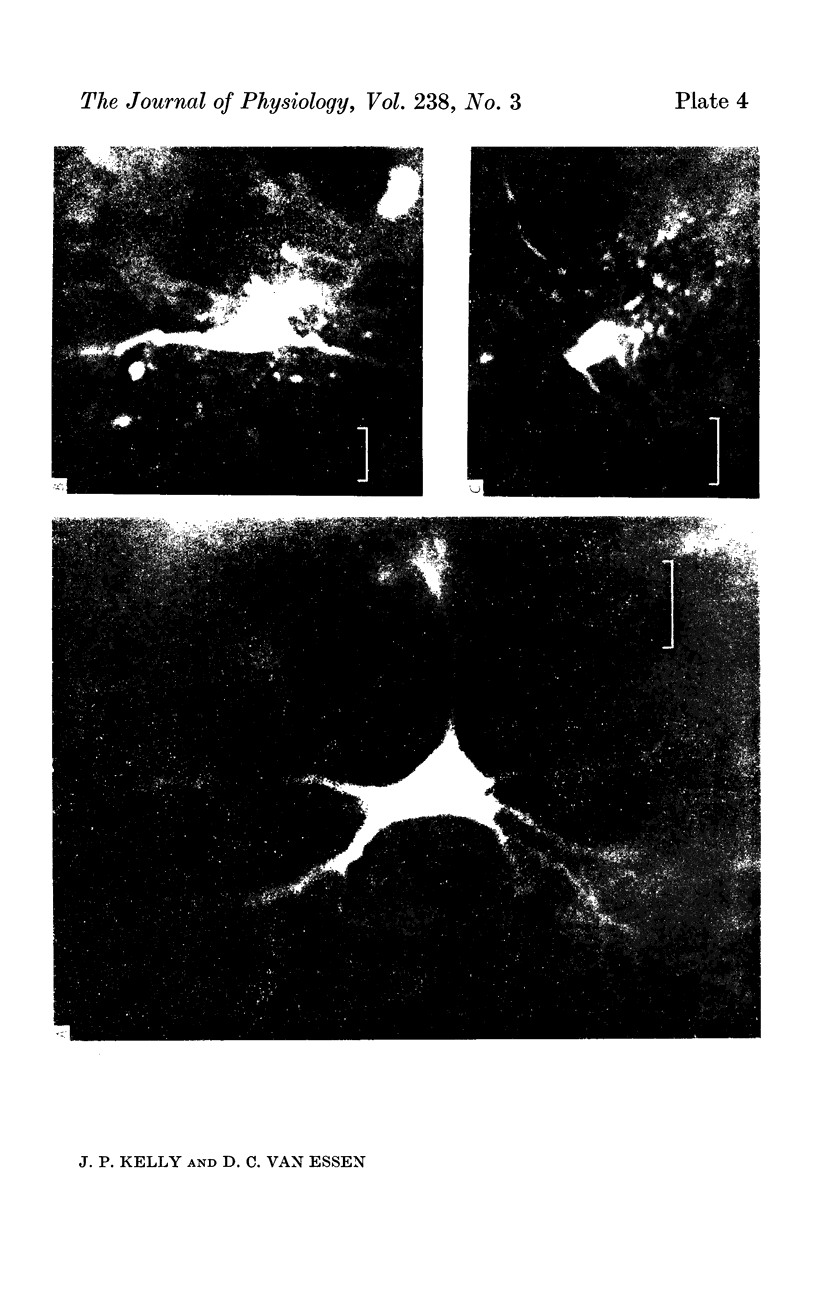
1. The organization of the visual cortex was studied with a technique that allows one to determine the physiology and morphology of individual cells. Micro-electrodes filled with the fluorescent dye Procion yellow were used to record intracellularly from cells in area 17 of the cat. The visual receptive field of each neurone was classified as simple, complex, or hypercomplex, and the cell was then stained by the iontophoretic injection of dye.
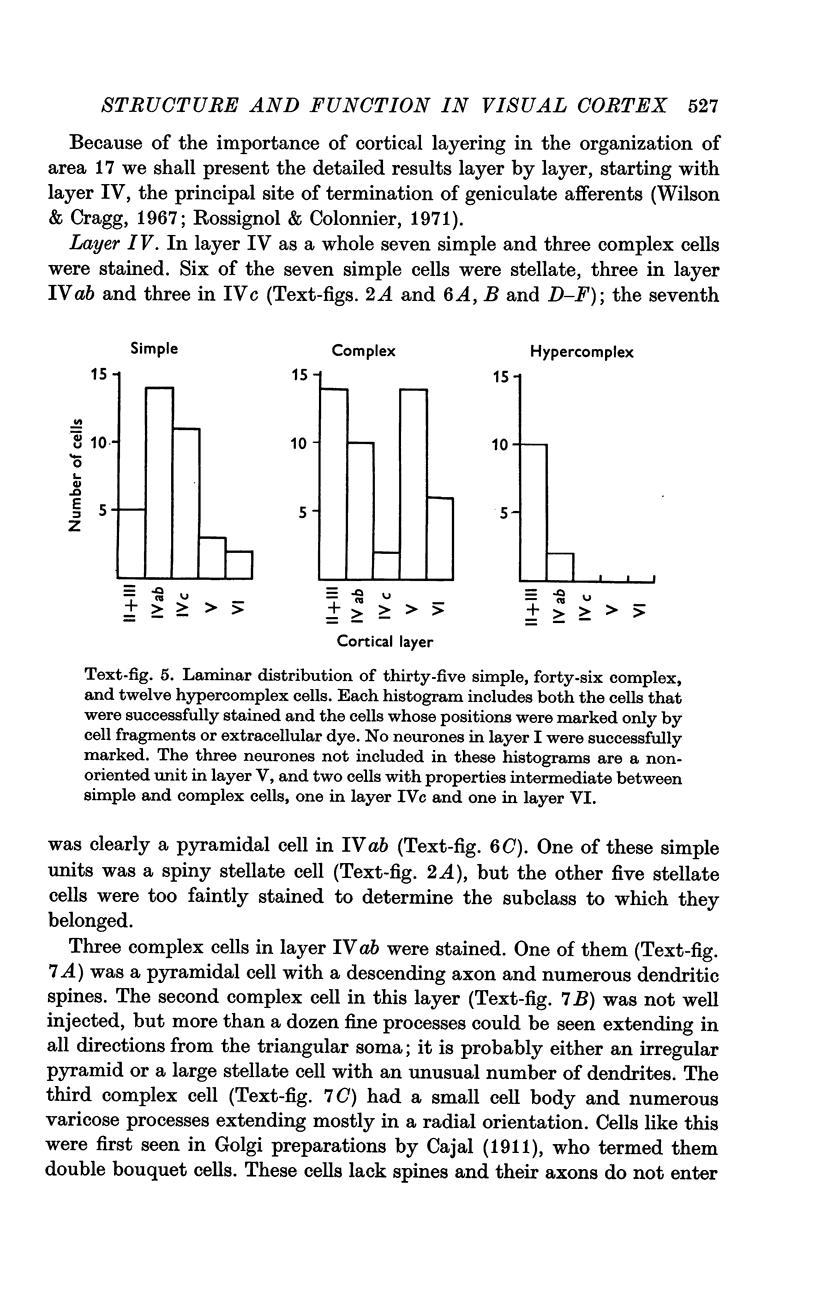
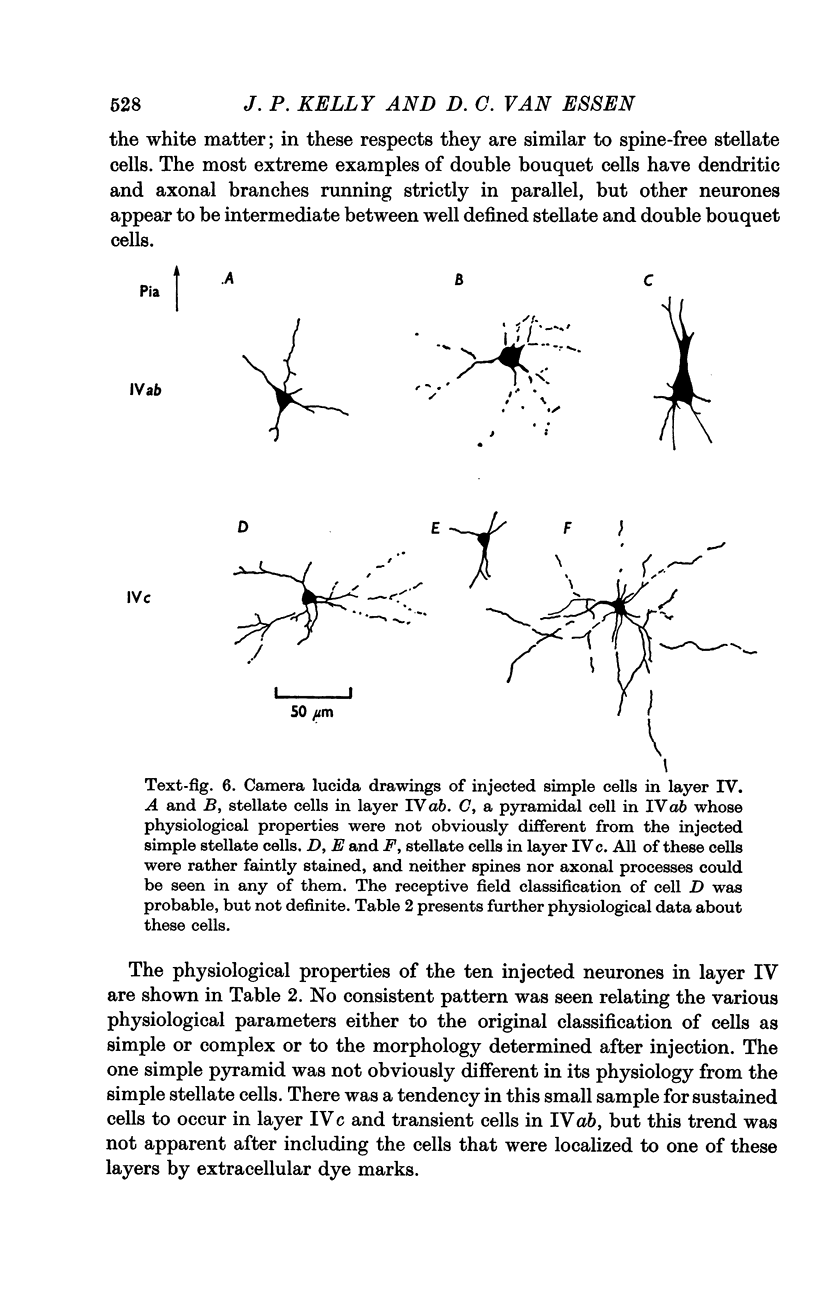
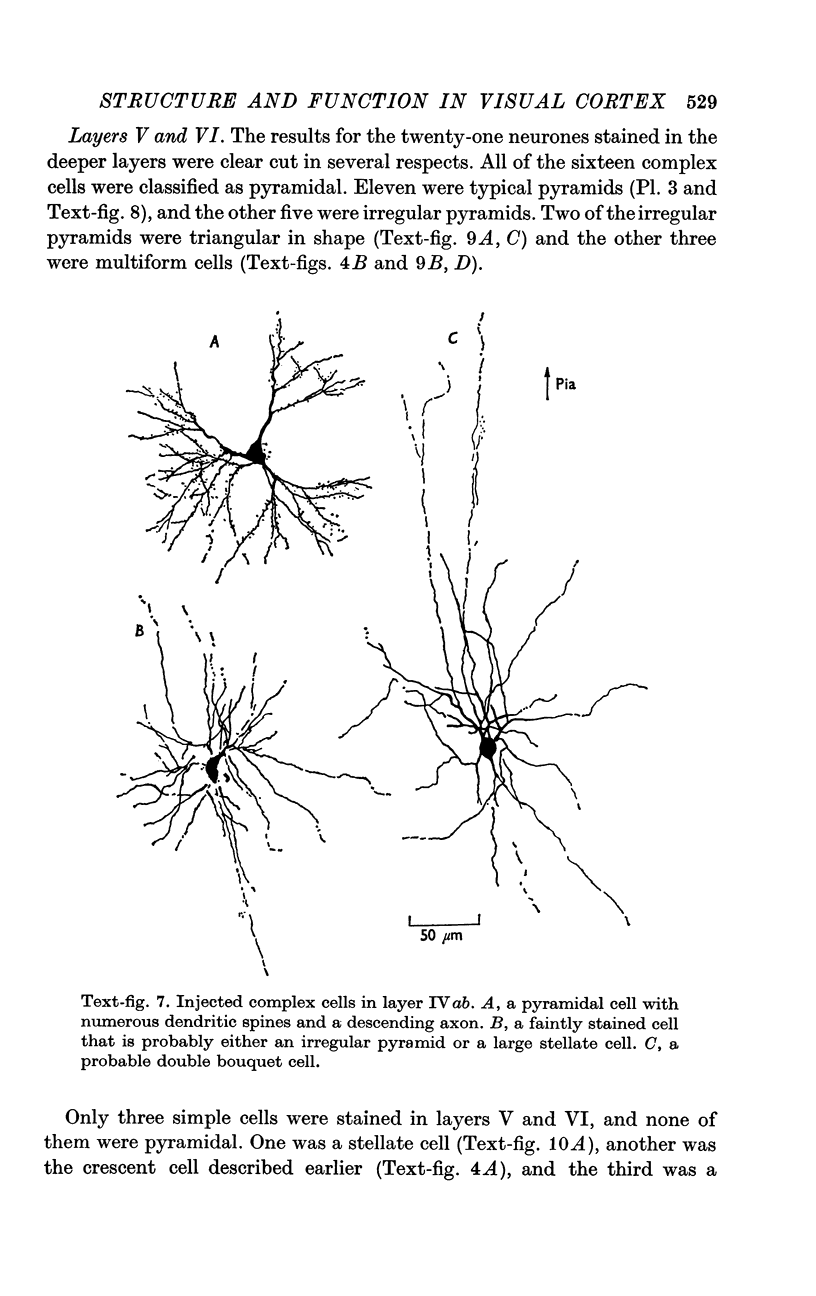
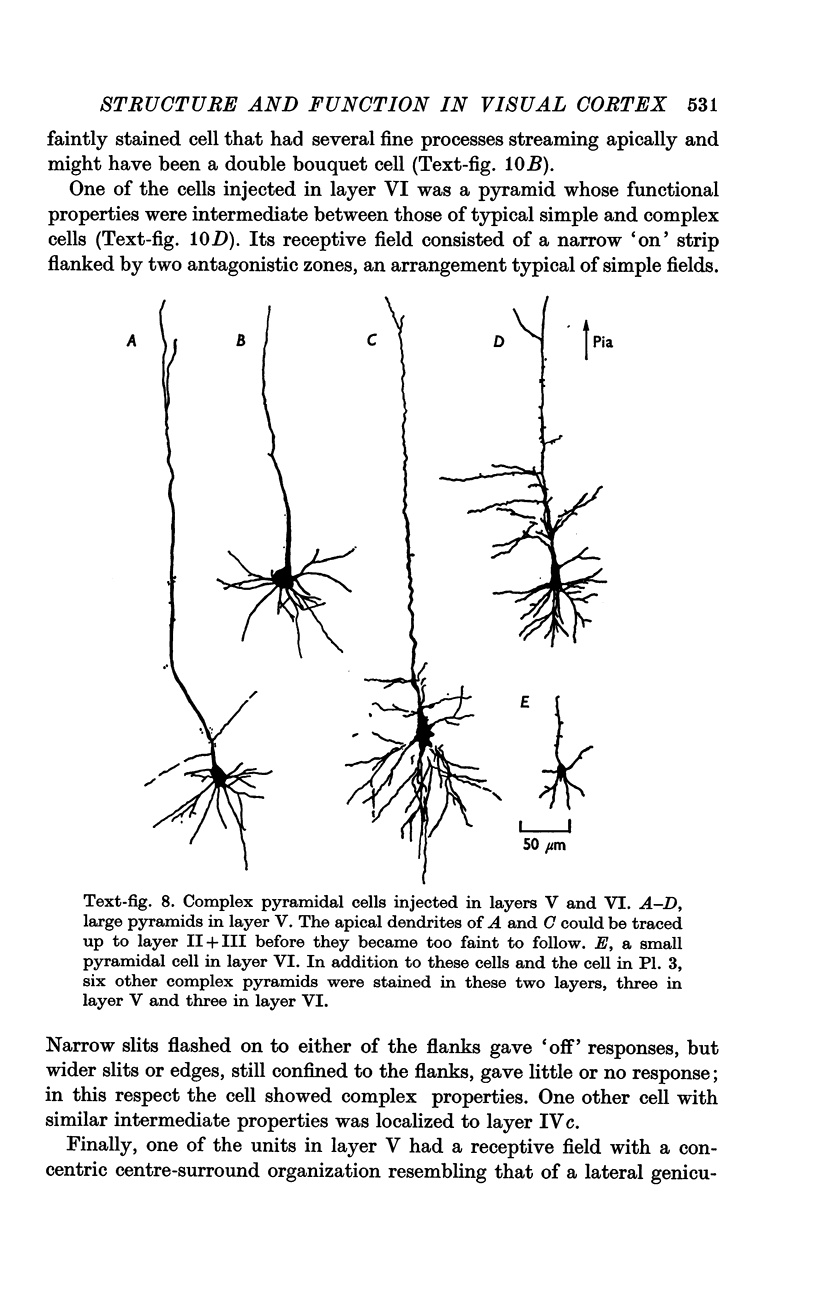
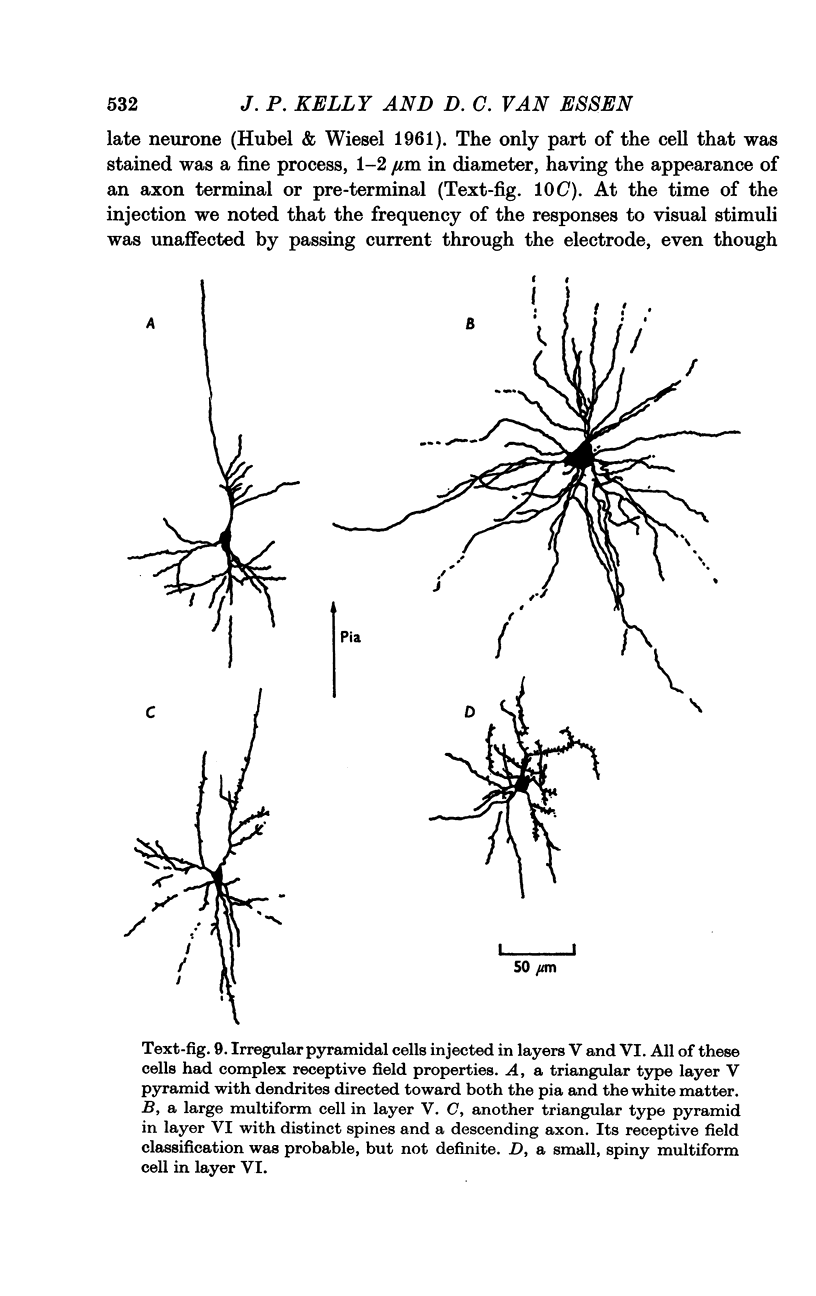
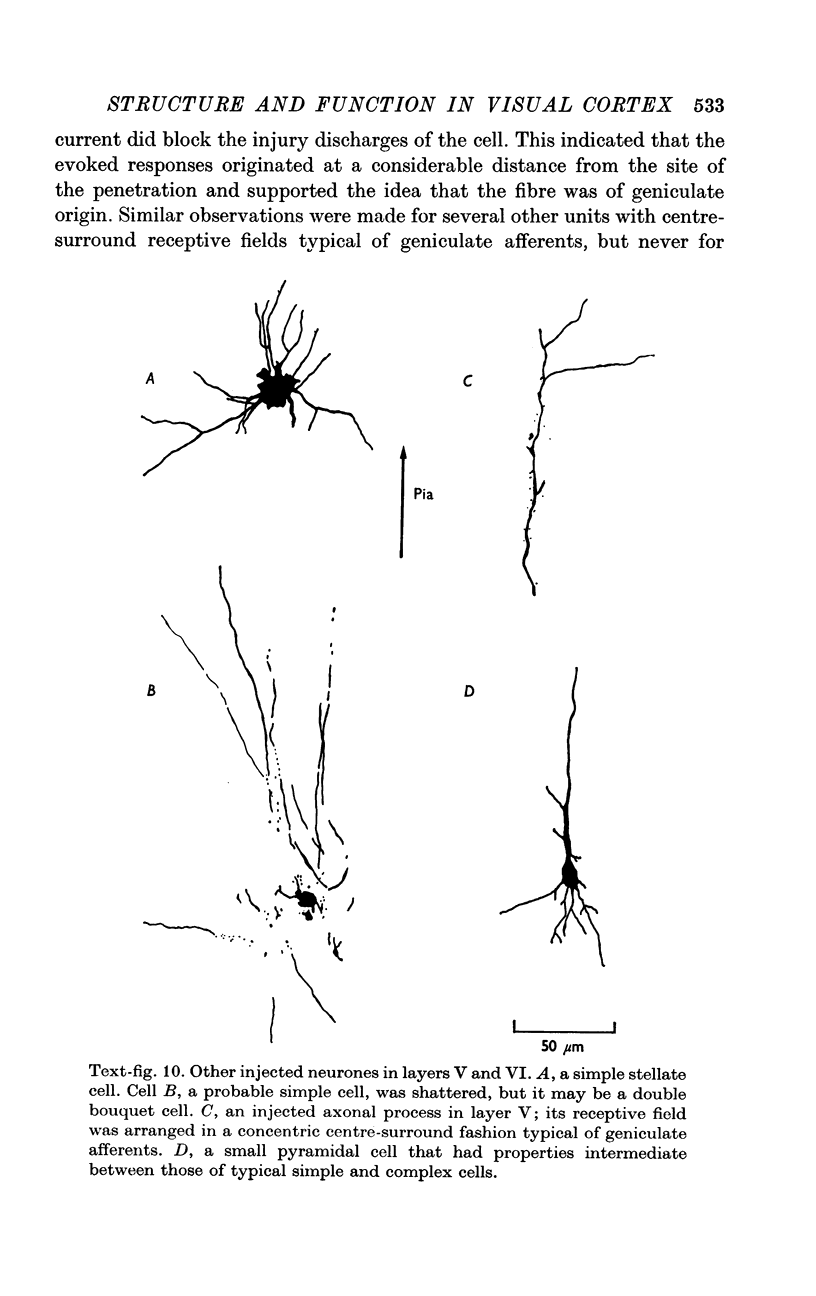
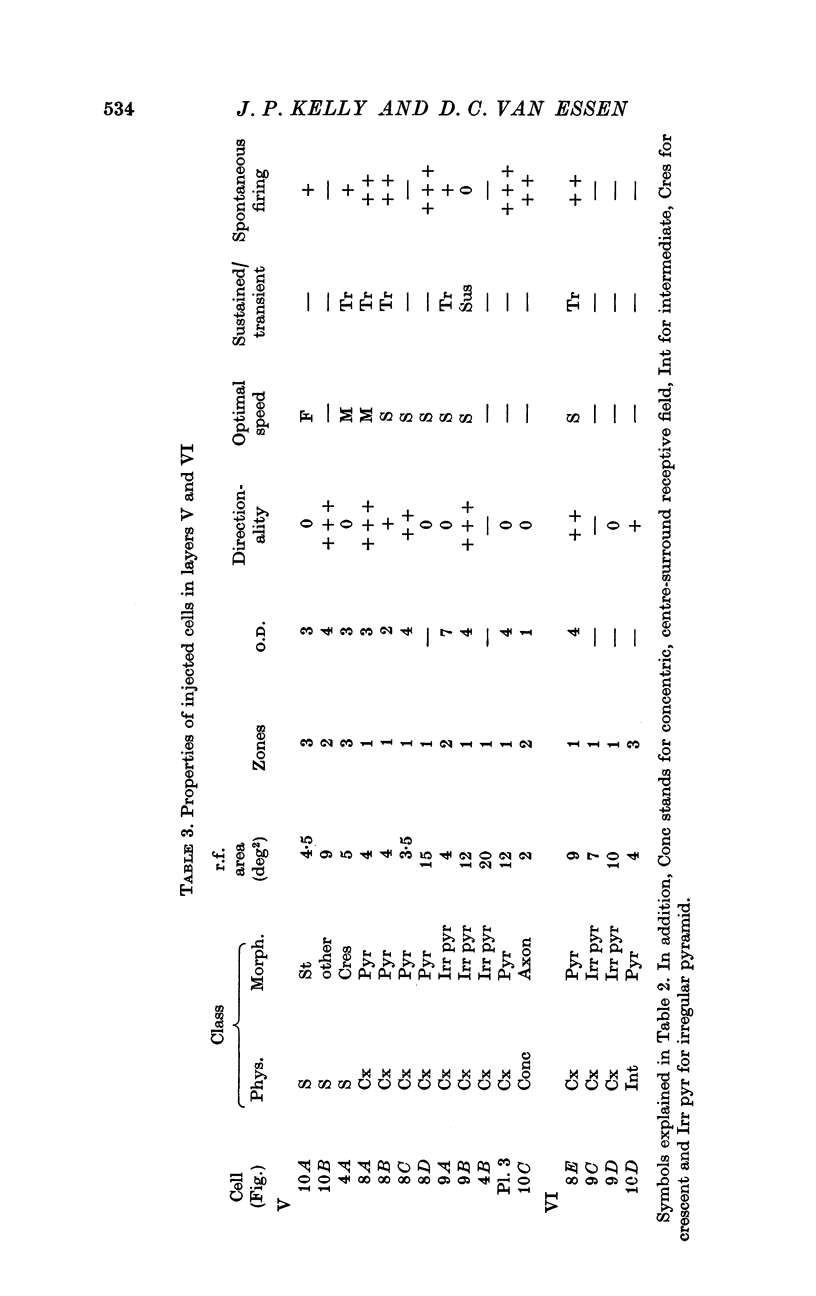
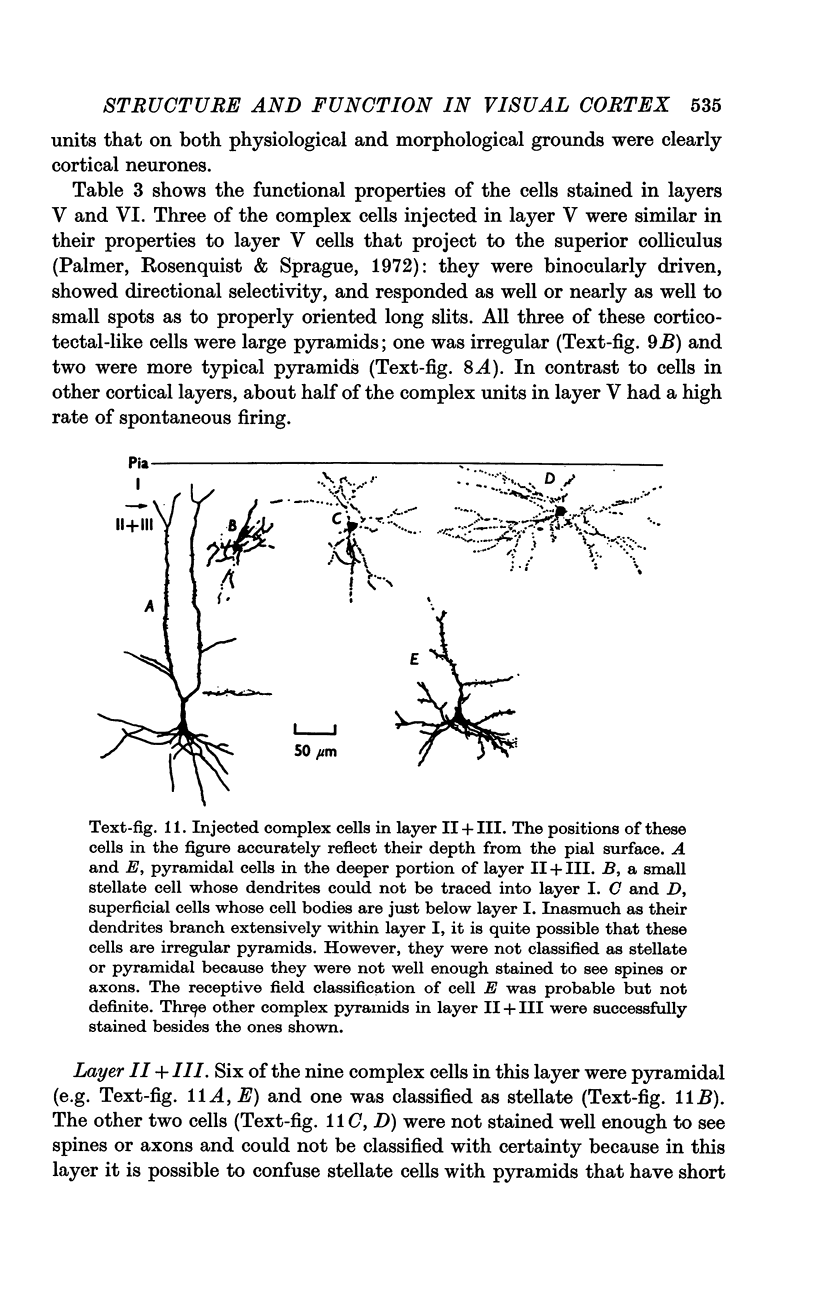
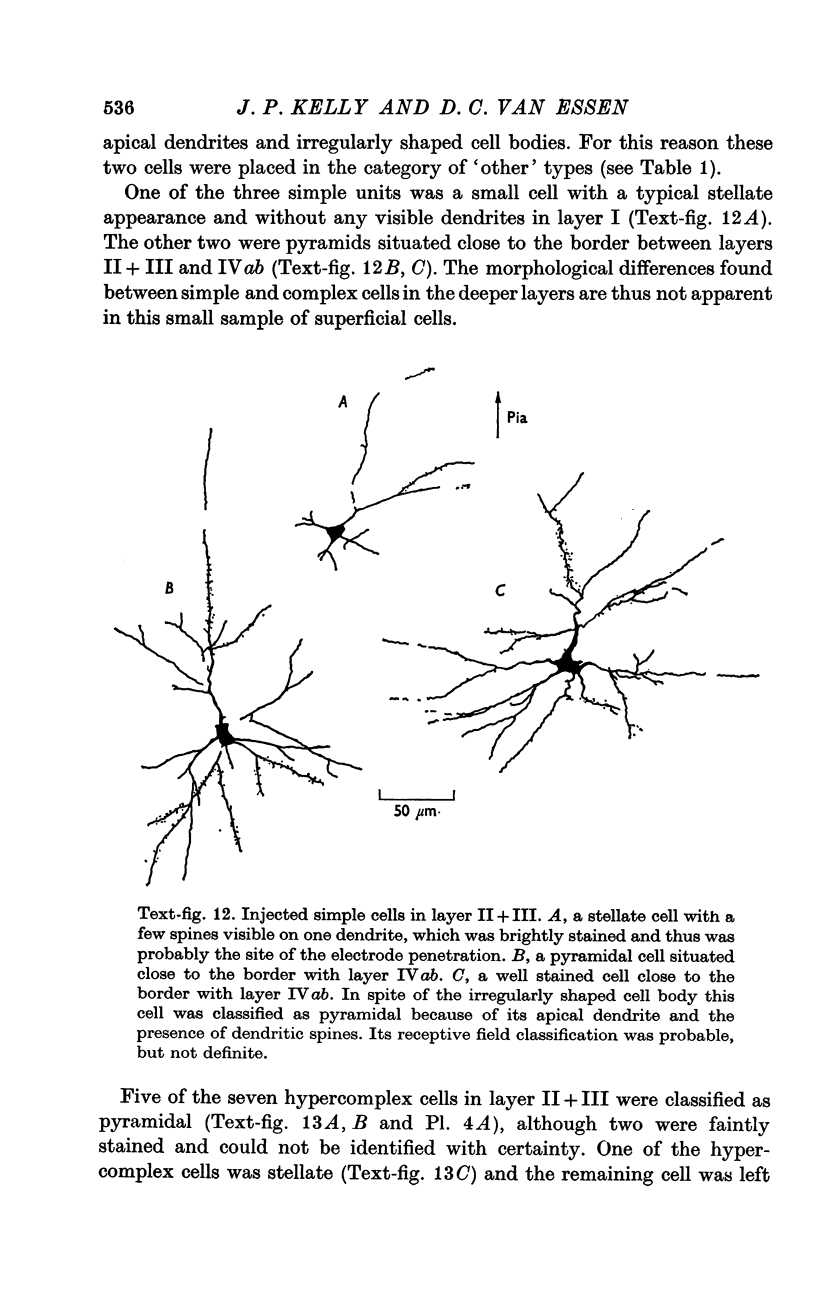
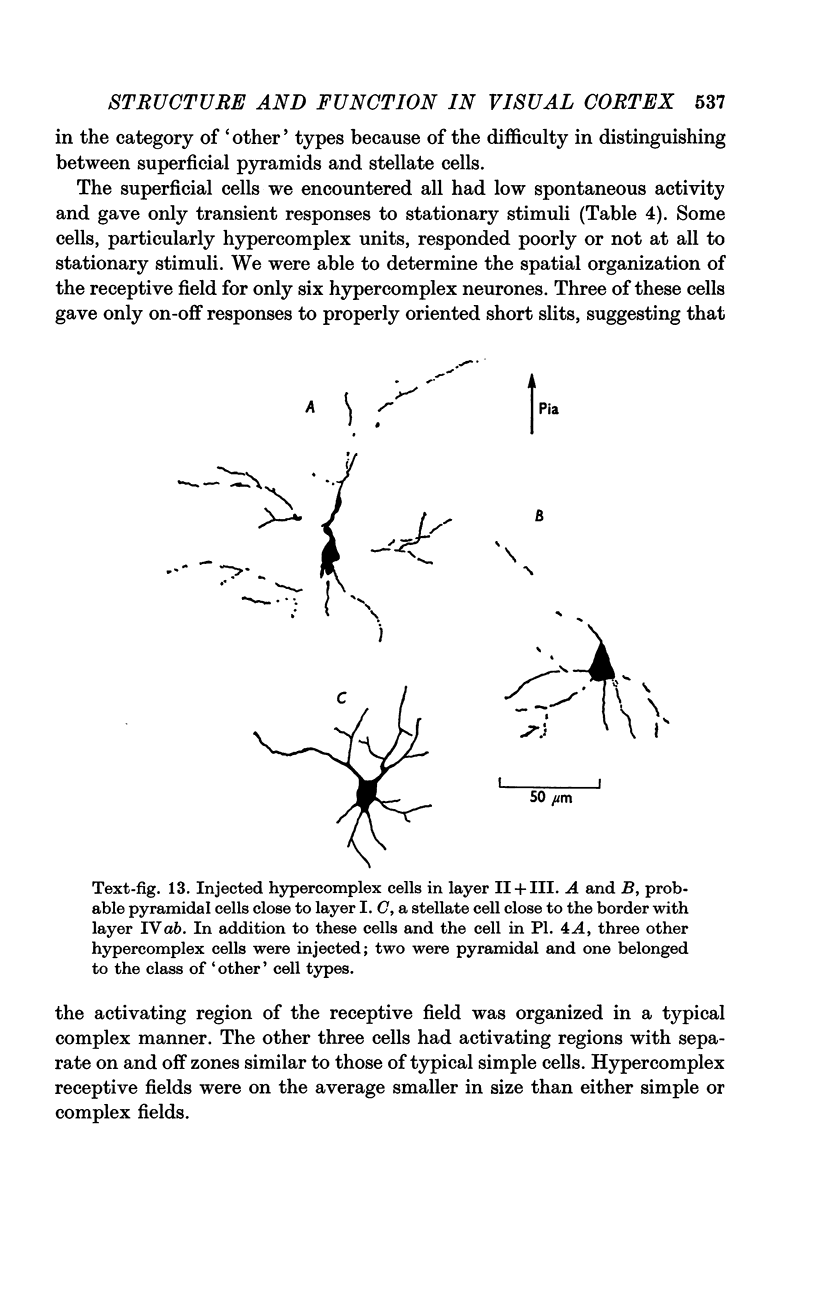
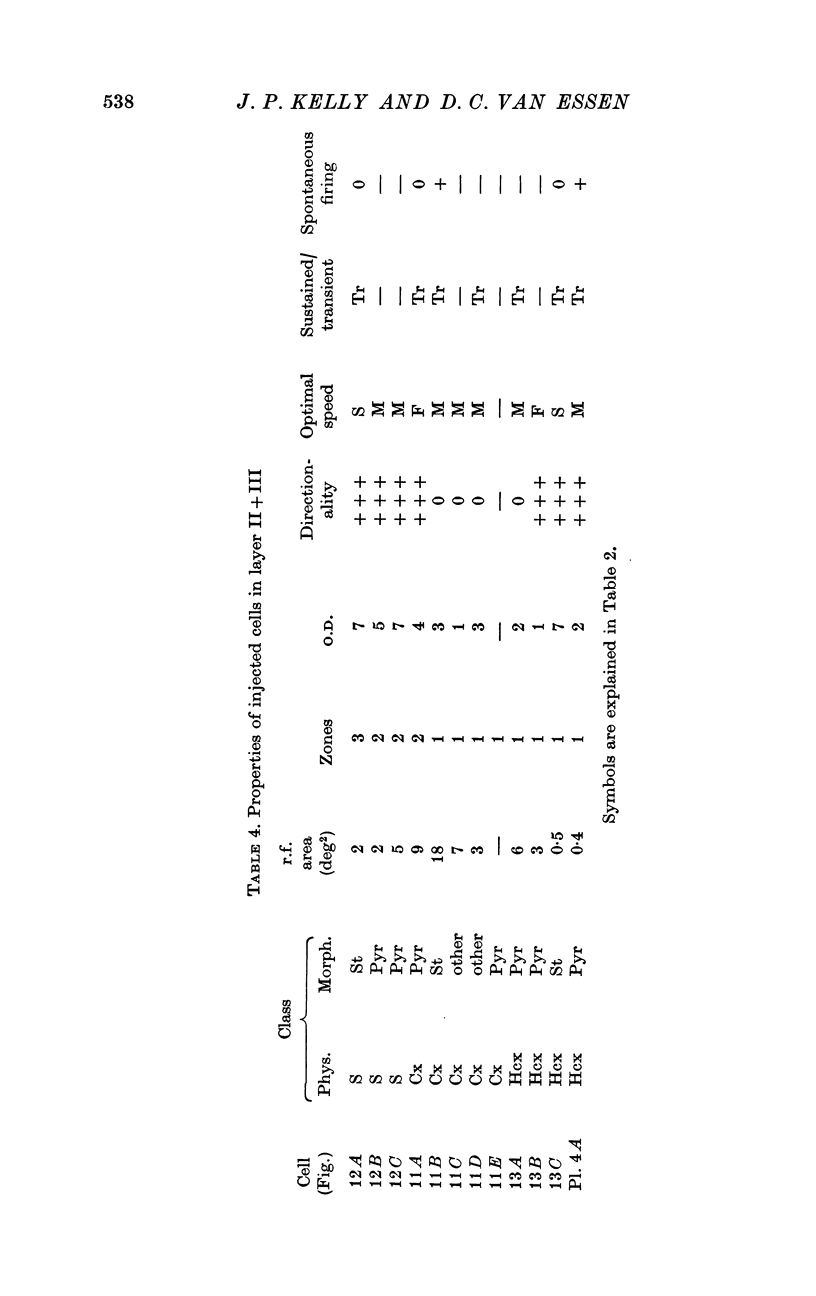
2. Fifty neurones were successfully examined in this way, and their structural features were compared to the varieties of cell types seen in Golgi preparations of area 17. The majority of simple units were stellate cells, whereas the majority of complex and hypercomplex units were pyramidal cells. Several neurones belonged to less common morphological types, such as double bouquet cells. Simple cells were concentrated in layer IV, hypercomplex cells in layer II + III, and complex cells in layers II + III, V and VI.
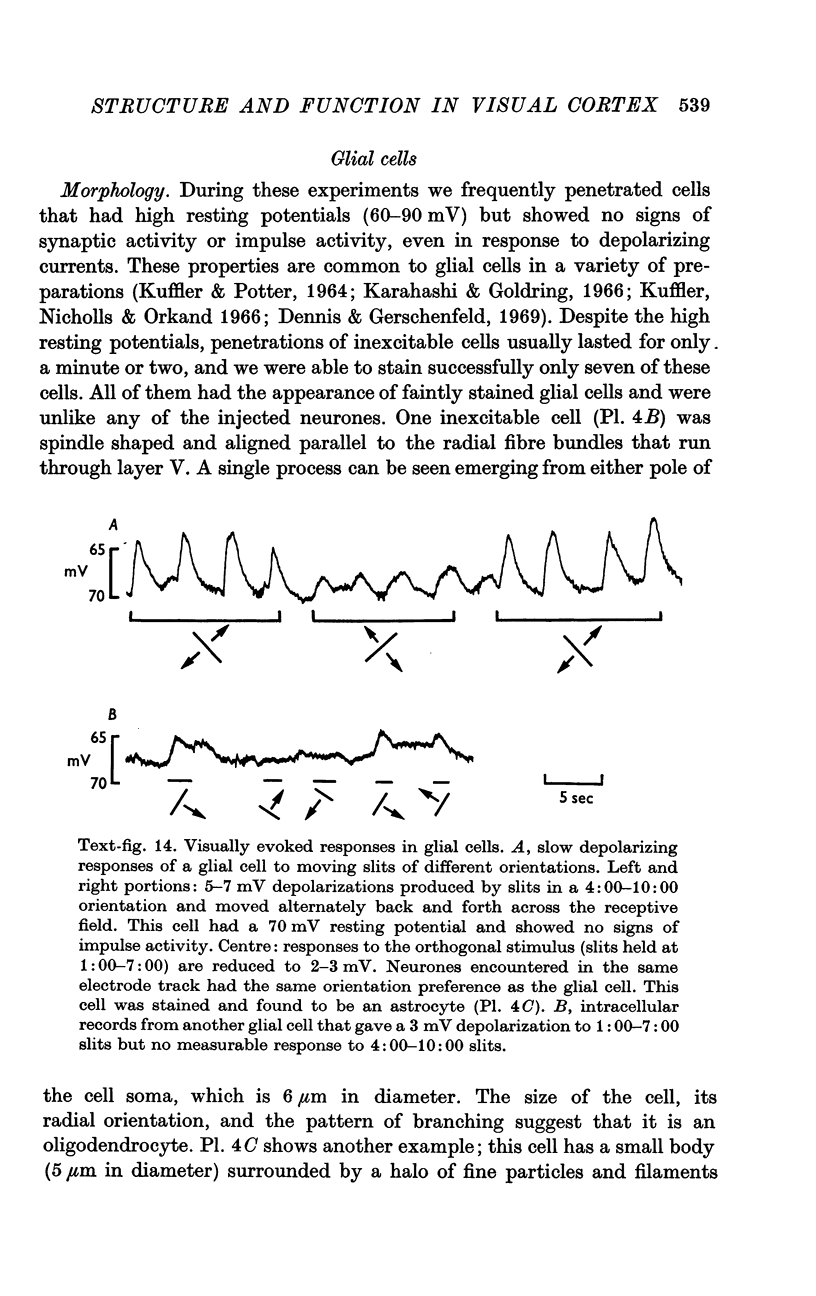
3. Electrically inexcitable cells that had high resting potentials but no impulse activity were stained and identified as glial cells. Glial cells responded to visual stimuli with slow graded depolarizations, and many of them showed a preference for a stimulus orientation similar to the optimal orientation for adjacent neurones.
4. The results show that there is a clear, but not absolute correlation between the major structural and functional classes of cells in the visual cortex. This approach, linking the physiological properties of a single cell to a given morphological type, will help in furthering our understanding of the cerebral cortex.
Full text
PDF




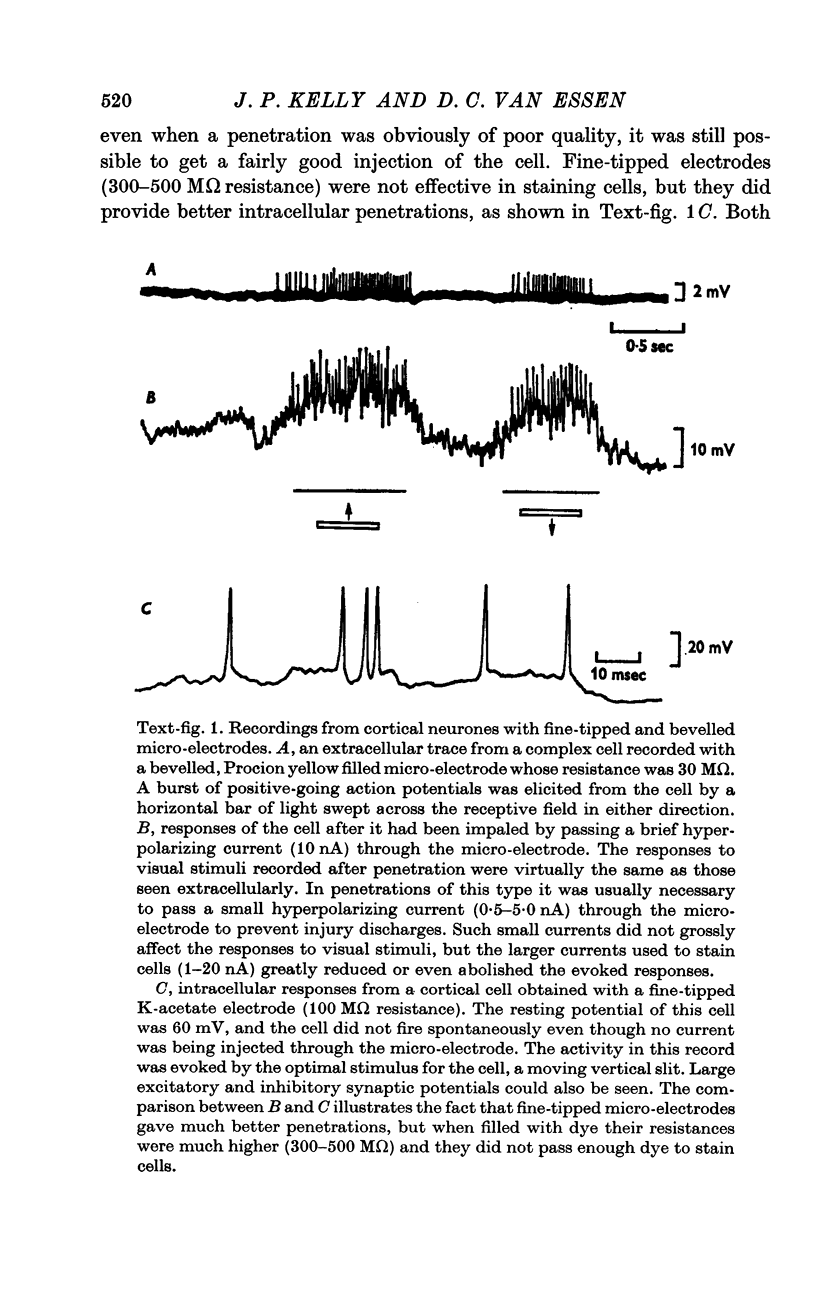












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Graubard K. Fluorescent staining of cat motoneurons in vivo with beveled micropipettes. Brain Res. 1970 Mar 17;18(3):565–568. doi: 10.1016/0006-8993(70)90143-5. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Gerschenfeld H. M. Some physiological properties of identified mammalian neuroglial cells. J Physiol. 1969 Jul;203(1):211–222. doi: 10.1113/jphysiol.1969.sp008860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Grossman R. G., Hampton T. Depolarization of cortical glial cells during electrocortical activity. Brain Res. 1968 Nov;11(2):316–324. doi: 10.1016/0006-8993(68)90027-9. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., POTTER D. D. GLIA IN THE LEECH CENTRAL NERVOUS SYSTEM: PHYSIOLOGICAL PROPERTIES AND NEURON-GLIA RELATIONSHIP. J Neurophysiol. 1964 Mar;27:290–320. doi: 10.1152/jn.1964.27.2.290. [DOI] [PubMed] [Google Scholar]
- Karahashi Y., Goldring S. Intracellular potentials from "idle" cells in cerebral cortex of cat. Electroencephalogr Clin Neurophysiol. 1966 Jun;20(6):600–607. doi: 10.1016/0013-4694(66)90024-1. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Yim G. K. Unresponsive cells in cerebral cortex. Brain Res. 1967 Dec;6(4):767–769. doi: 10.1016/0006-8993(67)90132-1. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. J Comp Neurol. 1973 Jul 1;150(1):53–85. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Morest D. K., Morest R. R. Perfusion-fixation of the brain with chrome-osmium solutions for the rapid Golgi method. Am J Anat. 1966 May;118(3):811–831. doi: 10.1002/aja.1001180309. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Slow depolarization in cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):869–878. doi: 10.1152/jn.1973.36.5.869. [DOI] [PubMed] [Google Scholar]
- Rossignol S., Colonnier M. A light microscope study of degeneration patterns in cat cortex after lesions of the lateral geniculate nucleus. Vision Res. 1971;Suppl 3:329–338. doi: 10.1016/0042-6989(71)90049-6. [DOI] [PubMed] [Google Scholar]
- SHOLL D. A. The organization of the visual cortex in the cat. J Anat. 1955 Jan;89(1):33–46. [PMC free article] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Sugaya E., Karahashi Y., Sugaya A., Haruki F. Intra- and extra-cellular potentials from "idle" cells in cerebral cortex of cat. Jpn J Physiol. 1971 Apr;21(2):149–157. doi: 10.2170/jjphysiol.21.149. [DOI] [PubMed] [Google Scholar]
- Van Essen D., Kelly J. Correlation of cell shape and function in the visual cortex of the cat. Nature. 1973 Feb 9;241(5389):403–405. doi: 10.1038/241403a0. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Cragg B. G. Projections from the lateral geniculate nucleus in the cat and monkey. J Anat. 1967 Sep;101(Pt 4):677–692. [PMC free article] [PubMed] [Google Scholar]
- le Gros Clark W. E. The cells of Meynert in the visual cortex of the monkey. J Anat. 1942 Jul;76(Pt 4):369–376.1. [PMC free article] [PubMed] [Google Scholar]