Abstract
1. Based on our ultrastructural investigations in monkeys, we report here a new concept as to the physiological mechanism of drainage of cerebrospinal fluid (c.s.f.) which would seem to bridge the gap between the two apparently opposing views of `closed' and `open' system.
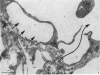
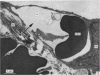
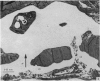
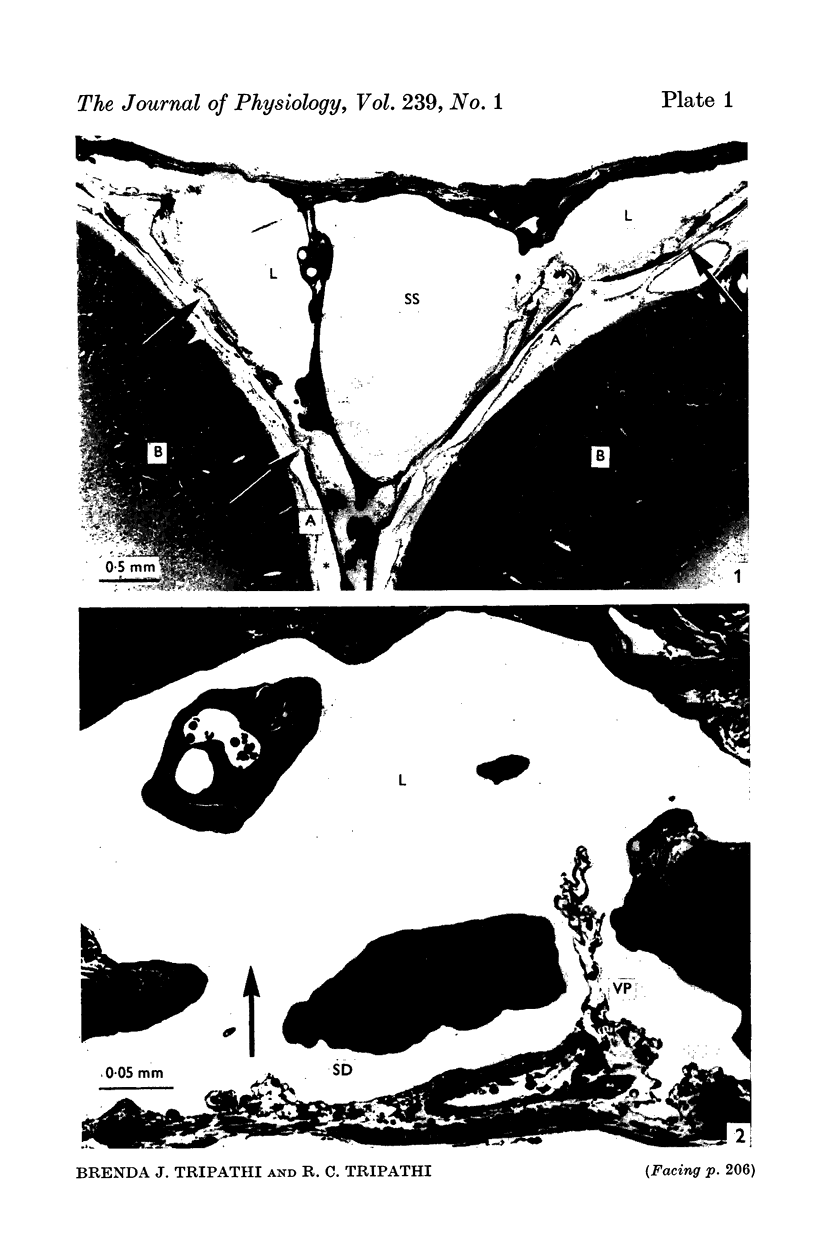
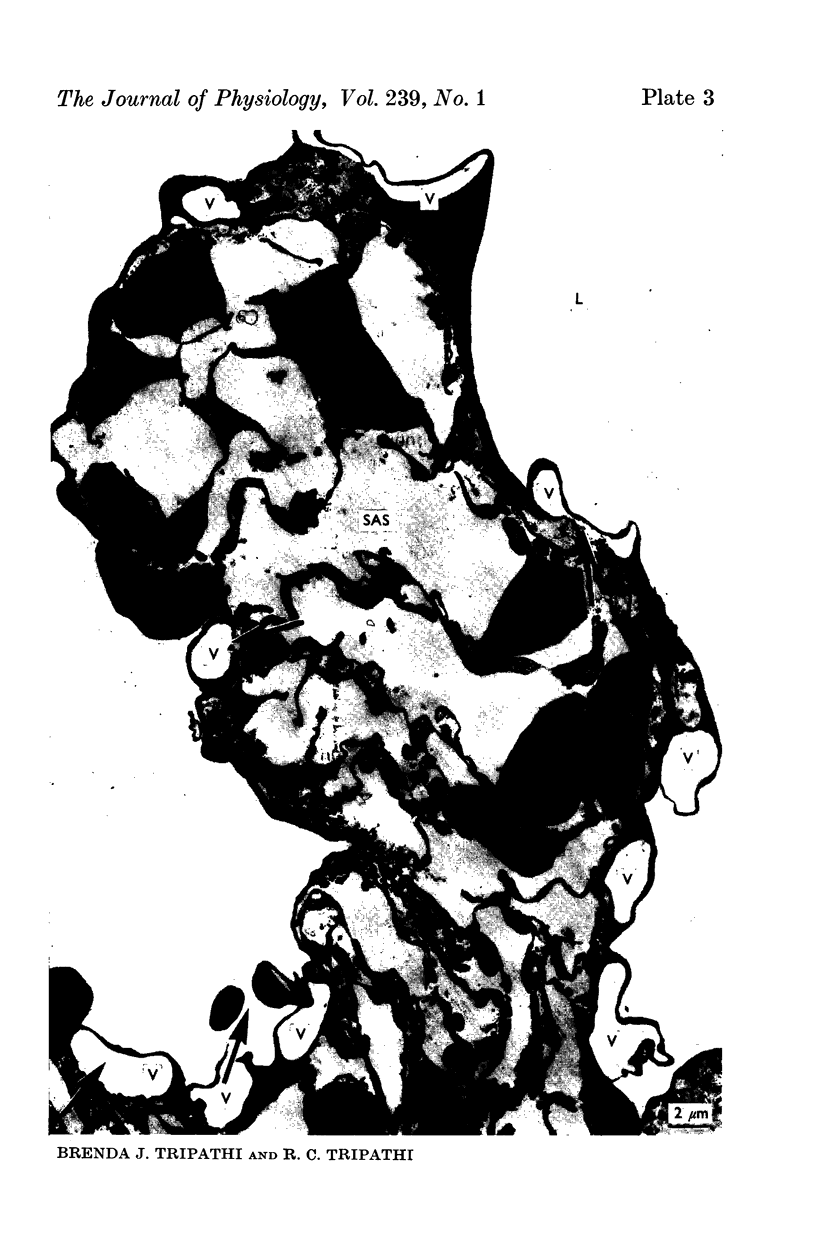
2. Our studies reveal the presence of an intact mesothelial lining of the arachnoid mater, including its villus-like projections and herniations into the dural sinus and its lacunae, adjacent cells being joined by tight junctions; in addition we have observed for the first time that many lining cells in the region of the superior sagittal sinus are characterized by unit membrane-bound, electron-optically empty giant vacuoles of several micrometres diameter. In one monkey with a fresh subarachnoid haemorrhage, many vacuoles were filled with plasma proteins and some contained intact blood corpuscles.
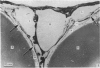
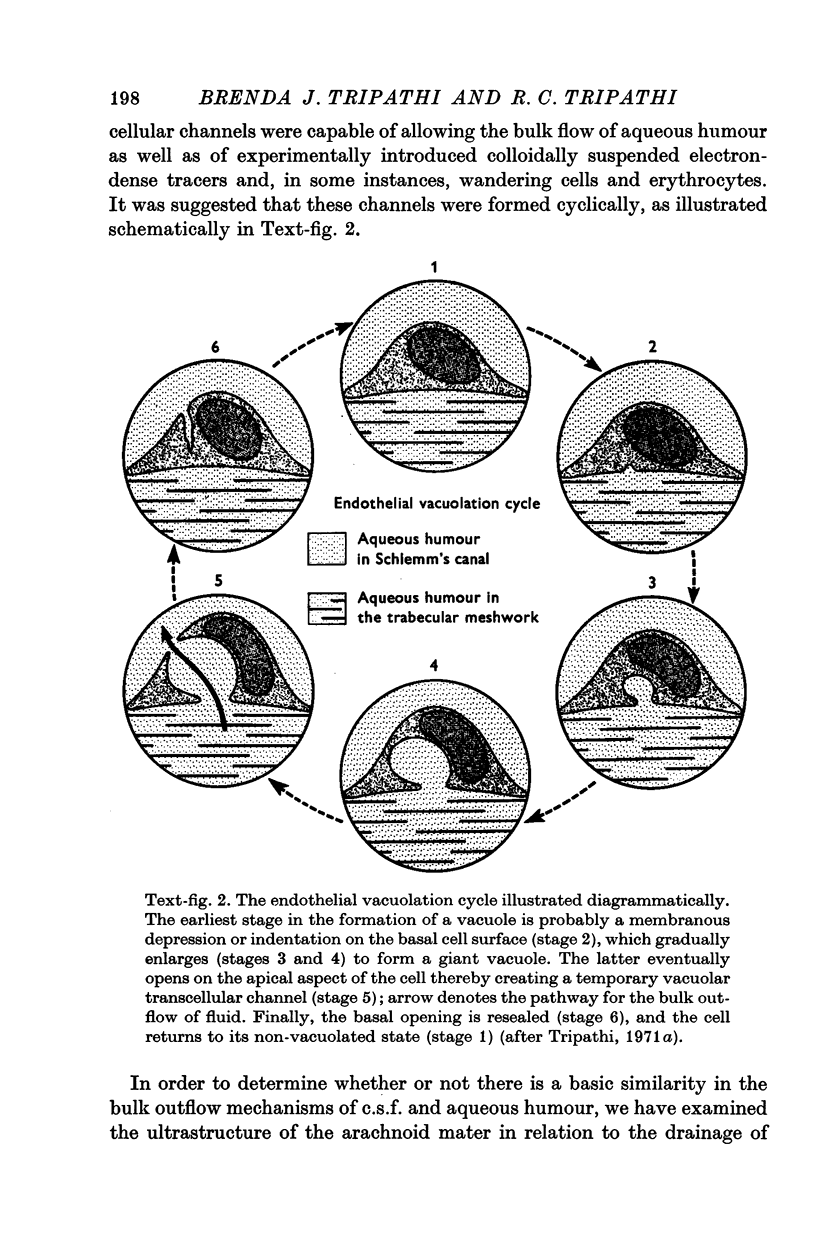
3. Serial section analysis showed that the vacuoles were in fact invaginations from the basal aspect of the cell surface and were evidently in direct communication with the c.s.f. in the subarachnoid space. Some vacuoles in addition showed openings on their apical surface thus constituting transcellular channels or pores. Basal openings up to 3·5 μm and apical openings up to 2·3 μm were seen.
4. It is postulated that vacuoles are stages in the formation of a dynamic system of transcellular pores which allow the bulk outflow of c.s.f. down a pressure gradient, and that the mesothelial vacuolation cycle, in providing the requisite number of transcellular pores across the mesothelial barrier at any time, is a controlling factor in the outflow of c.s.f. and in the maintenance of its fluid-pressure within the subarachnoid space.
5. The basic similarity between the bulk flow of the aqueous humour and c.s.f. from the anatomically closed cavities of the anterior chamber and the subarachnoid space, respectively, is underlined.
6. The present study provides further support for our hypothesis that the bulk outflow of fluid, via a dynamic system of transcellular pores formed by gradually enlarging membranous surface infoldings in a single cell, termed as giant vacuoles, is a fundamental biological process not hitherto described.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alksne J. F., Lovings E. T. Functional ultrastructure of the arachnoid villus. Arch Neurol. 1972 Nov;27(5):371–377. doi: 10.1001/archneur.1972.00490170003002. [DOI] [PubMed] [Google Scholar]
- COURTICE F. C., SIMMONDS W. J. The removal of protein from the subarachnoid space. Aust J Exp Biol Med Sci. 1951 Jul;29(4):255–263. doi: 10.1038/icb.1951.30. [DOI] [PubMed] [Google Scholar]
- DAVSON H. The rates of disappearance of substances injected into the subarachnoid space of rabbits. J Physiol. 1955 May 27;128(2):52–3P. [PubMed] [Google Scholar]
- Davson H., Domer F. R., Hollingsworth J. R. The mechanism of drainage of the cerebrospinal fluid. Brain. 1973 Jun;96(2):329–336. doi: 10.1093/brain/96.2.329. [DOI] [PubMed] [Google Scholar]
- Davson H. Dynamic aspects of cerebrospinal fluid. Dev Med Child Neurol Suppl. 1972;27:1–16. doi: 10.1111/j.1469-8749.1972.tb09767.x. [DOI] [PubMed] [Google Scholar]
- PROCKOP L. D., SCHANKER L. S., BRODIE B. B. Passage of lipid-insoluble substances from cerebrospinal fluid to blood. J Pharmacol Exp Ther. 1962 Mar;135:266–270. [PubMed] [Google Scholar]
- SIMMONDS W. J. The absorption of labelled erythrocytes from the subarachnoid space in rabbits. Aust J Exp Biol Med Sci. 1953 Feb;31(1):77–83. doi: 10.1038/icb.1953.10. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Mechanism of the aqueous outflow across the trabecular wall of Schlemm's canal. Exp Eye Res. 1971 Jan;11(1):116–121. doi: 10.1016/s0014-4835(71)80073-8. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. The mechanism of aqueous outflow in birds. I. An ultrastructural study of normal eyes. Exp Eye Res. 1973 Mar;15(3):409–423. doi: 10.1016/0014-4835(73)90157-7. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. The mechanism of aqueous outflow in birds. II. An ultrastructural study of perfused eyes. Exp Eye Res. 1973 Mar;15(3):425–434. doi: 10.1016/0014-4835(73)90158-9. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. The mechanism of aqueous outflow in lower mammals. Exp Eye Res. 1972 Jul;14(1):73–79. doi: 10.1016/0014-4835(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of Schlemm's canal in relation to aqueous outflow. Exp Eye Res. 1968 Jul;7(3):335–341. doi: 10.1016/s0014-4835(68)80047-8. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of the arachnoid mater in relation to outflow of cerebrospinal fluid. A new concept. Lancet. 1973 Jul 7;2(7819):8–11. doi: 10.1016/s0140-6736(73)91945-4. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of the exit pathway of the aqueous in lower mammals. (A preliminary report on the "angular aqueous plexus"). Exp Eye Res. 1971 Nov;12(3):311–314. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- WELCH K., FRIEDMAN V. The cerebrospinal fluid valves. Brain. 1960 Sep;83:454–469. doi: 10.1093/brain/83.3.454. [DOI] [PubMed] [Google Scholar]
- WELCH K., POLLAY M. Perfusion of particles through arachnoid villi of the monkey. Am J Physiol. 1961 Oct;201:651–654. doi: 10.1152/ajplegacy.1961.201.4.651. [DOI] [PubMed] [Google Scholar]