Abstract
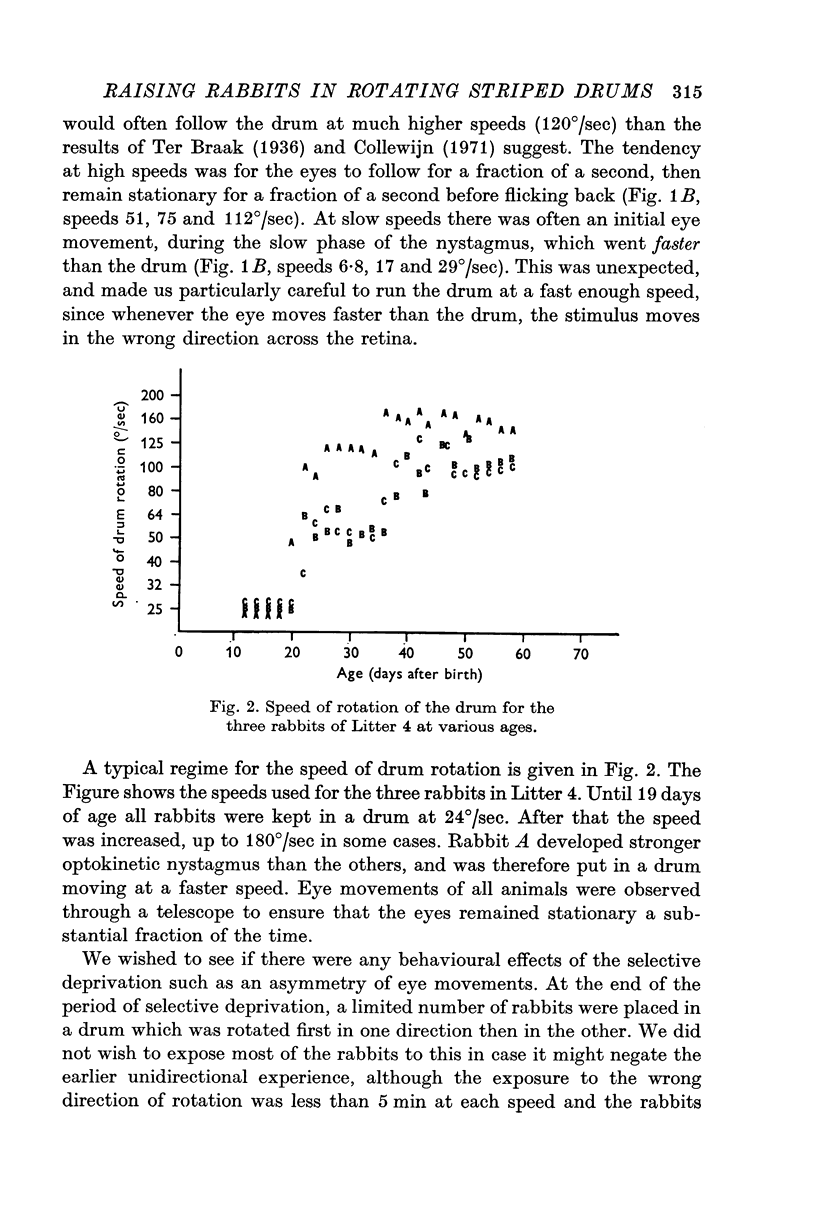
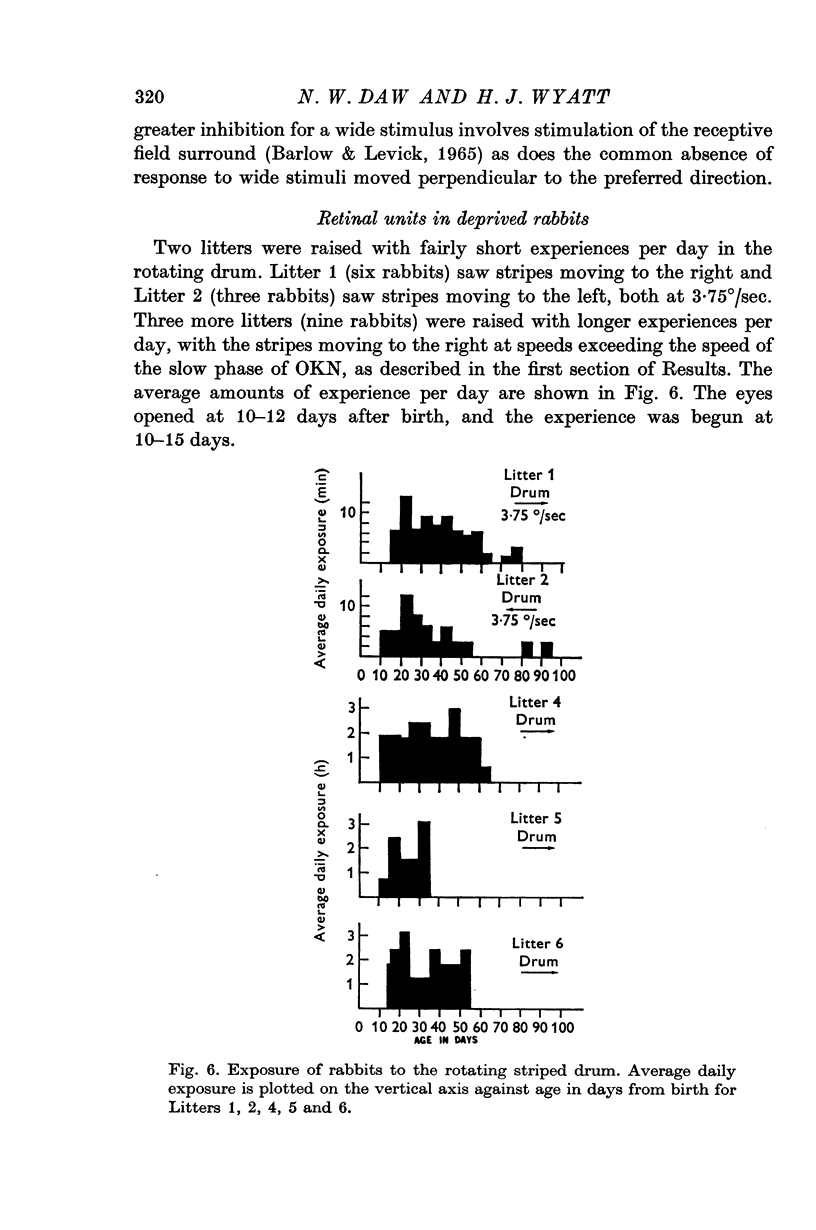
1. Rabbits were raised inside drums with vertical stripes painted on the inside. The rabbits were held stationary while the drum rotated continually around them: rotation was always in the same direction for any one animal. Rabbits in one litter were put in the drum for 15 min/day from 10-15 days after birth to about 60 days after birth, with the drum rotating to the right. Rabbits in another litter were put in for 15 min/day with the drum moving left. Rabbits in three other litters were put in for 2-3 hr/day with the drum moving right. All rabbits were kept in the dark when not in the drum.
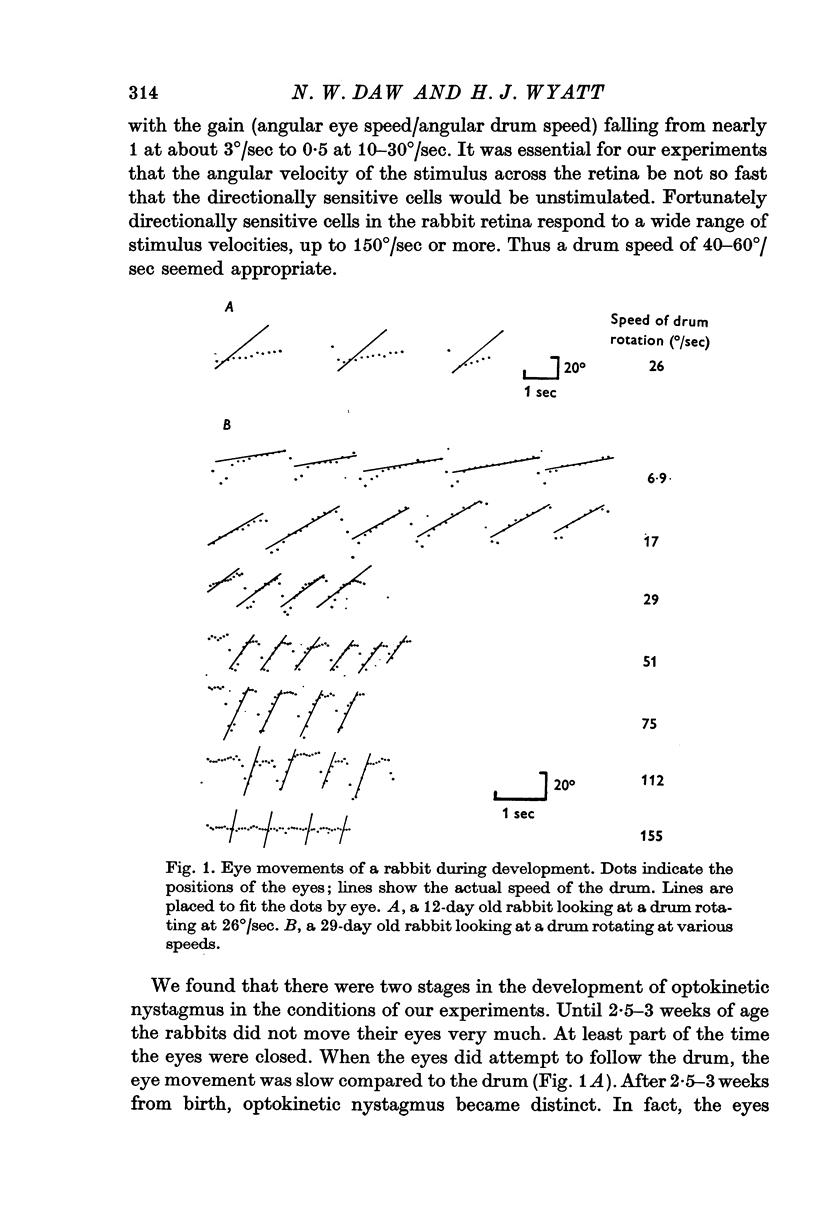
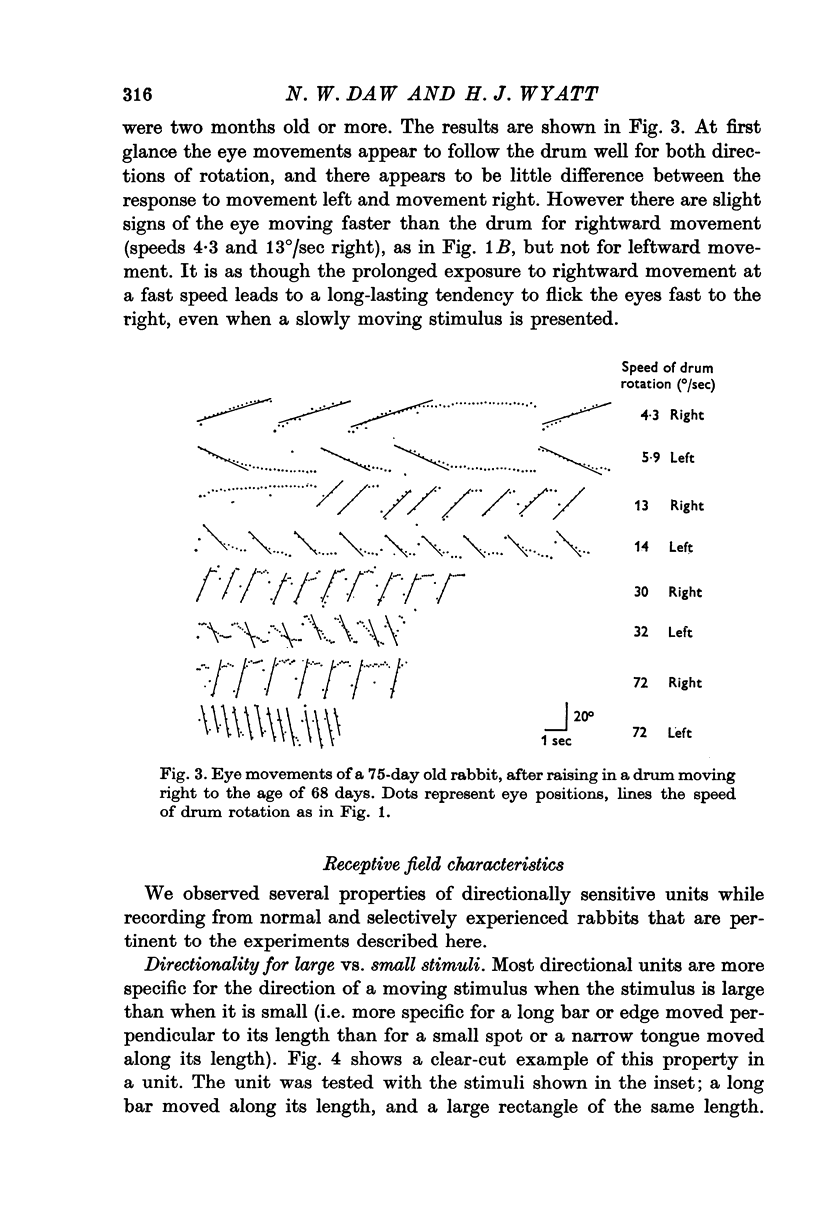
2. Optokinetic nystagmus was measured by photographing eye movements during drum rotation at various stages of development. The response to rotation in both directions was measured in a few adult animals. Only small differences were found in the adult animals between optokinetic nystagmus in response to a drum moving right compared to a drum moving left.
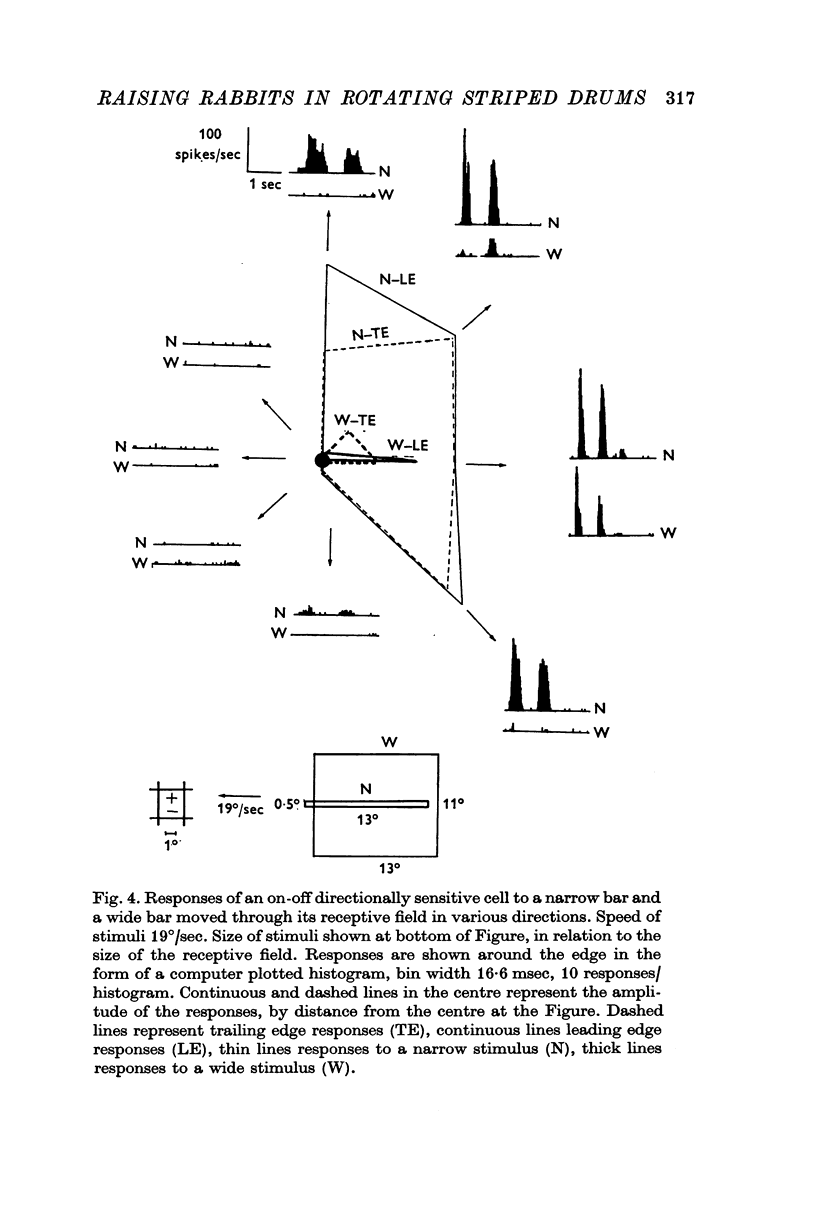
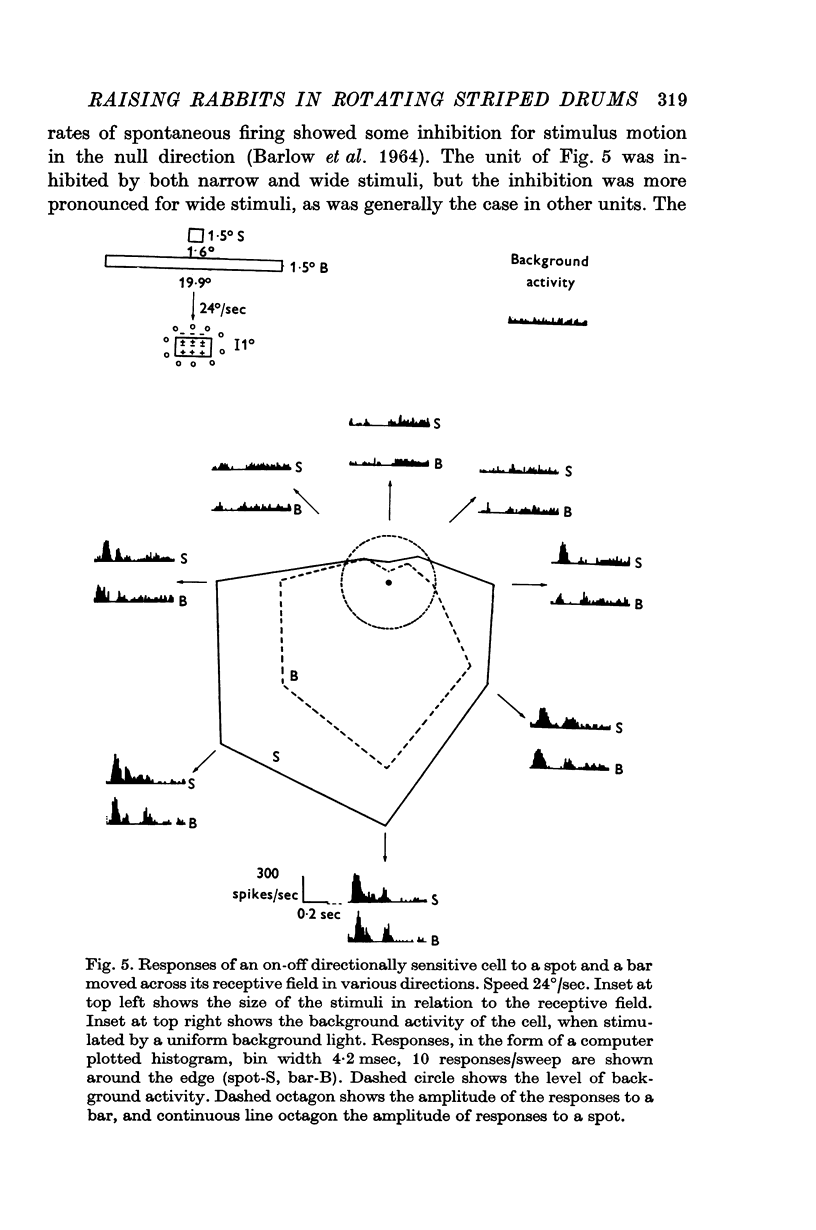
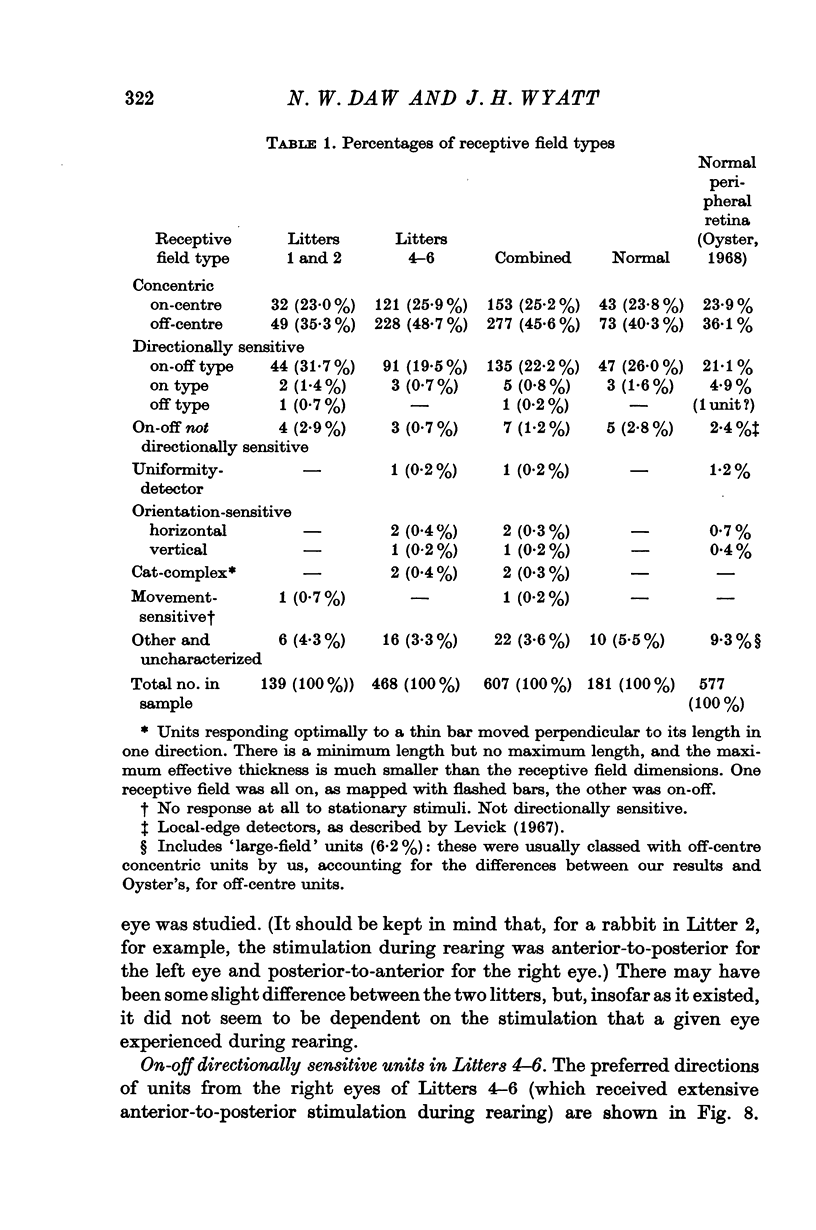
3. Recordings were made from ganglion cells in the retina and their receptive fields were mapped. A total of 607 cells from deprived rabbits were analysed. The percentages of on-centre and off-centre centre-surround types, on-off directionally sensitive types, and on-directionally sensitive types were not significantly different from normal.
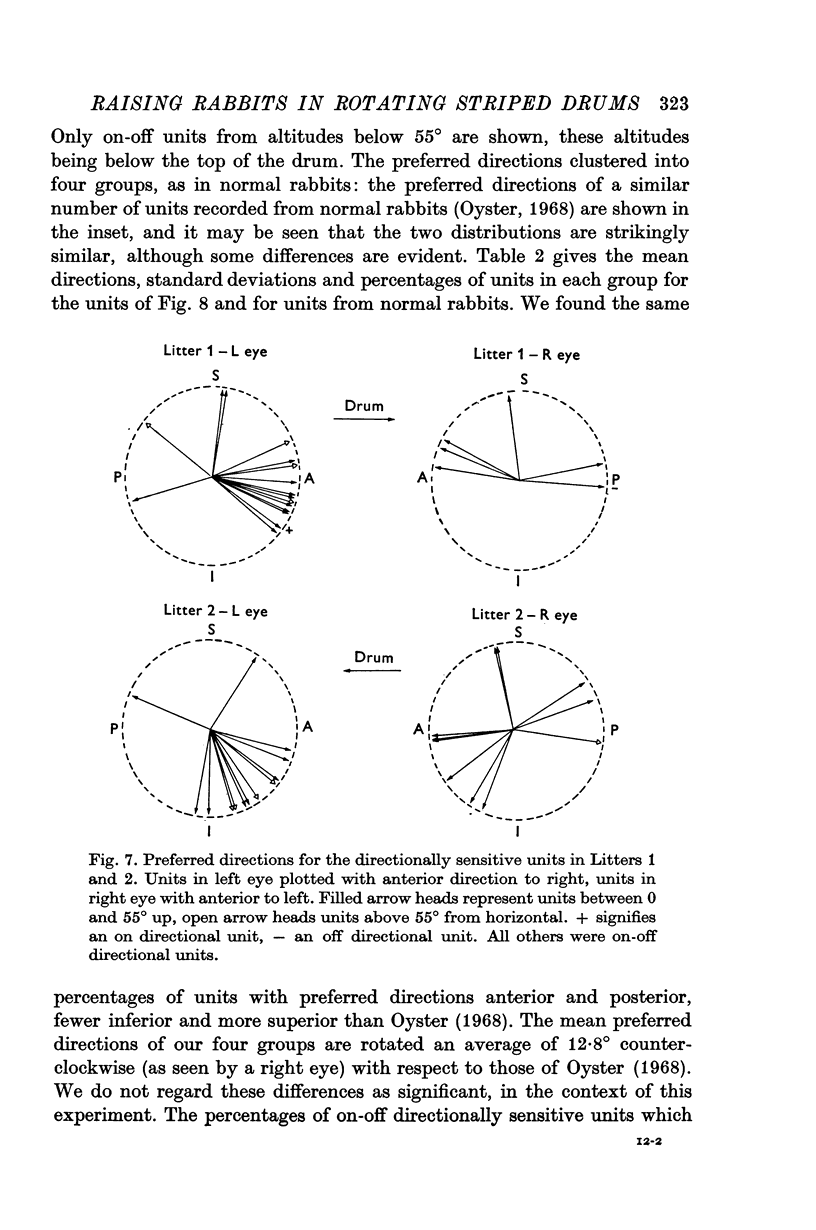
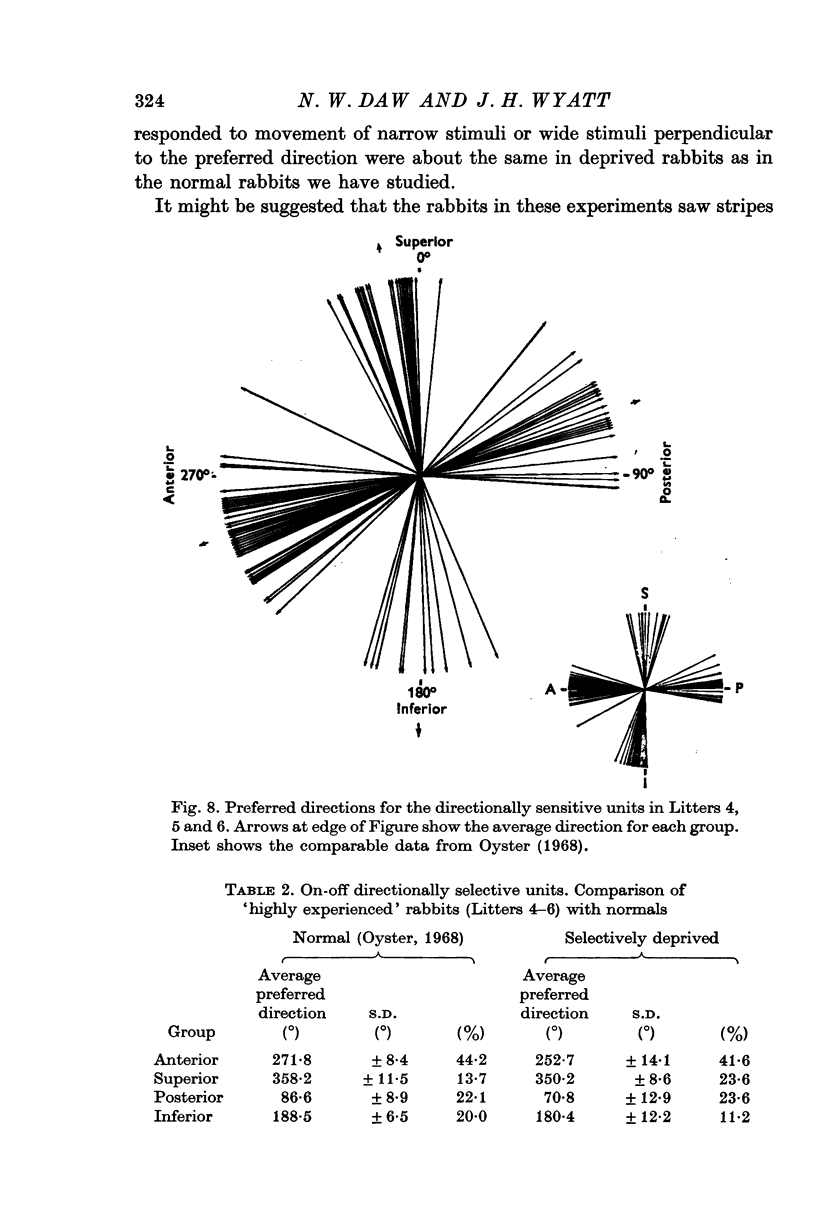
4. The percentages of directionally sensitive cells responding in the anterior, posterior, superior and inferior directions were normal. The fall-off in sensitivity for these cells with change in direction from the preferred direction was normal.
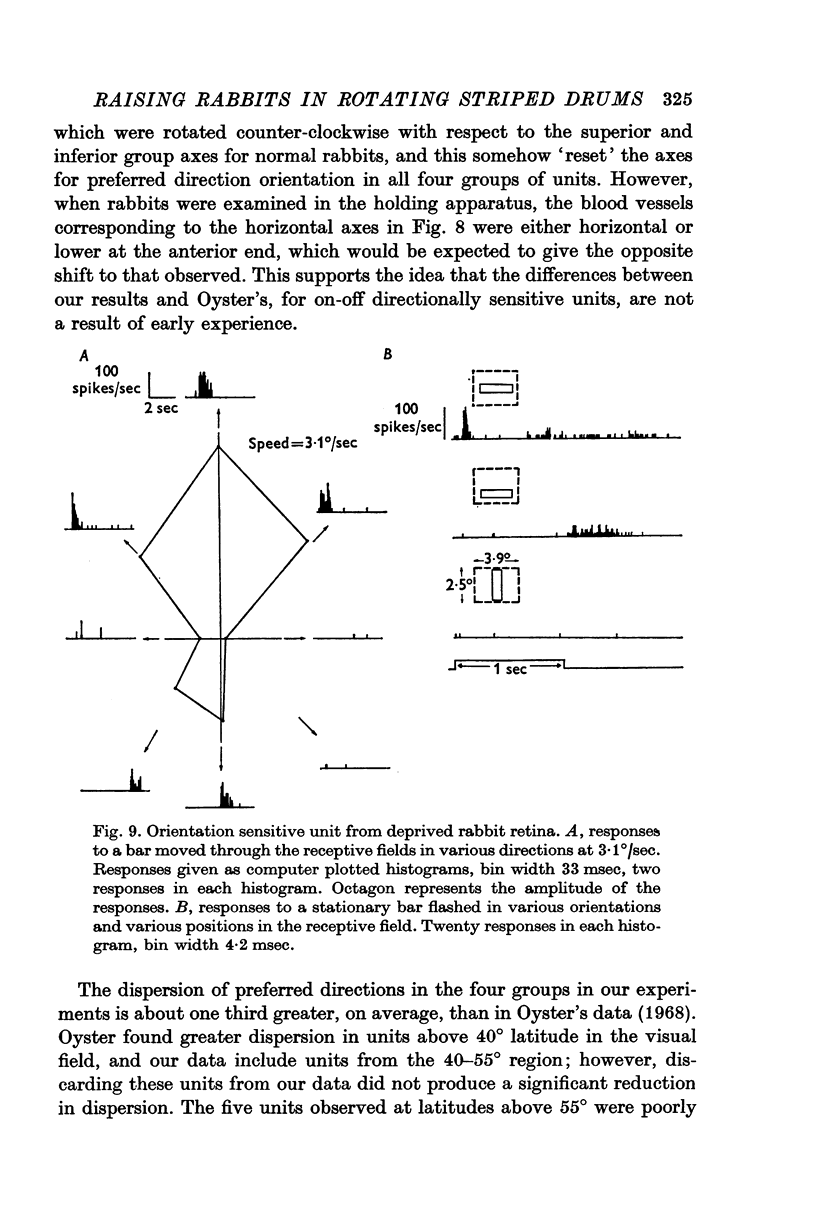
5. A few orientation sensitive cells were found responding to horizontally oriented bars.
6. We conclude that this selective deprivation of rabbits had little effect on the optokinetic response and no effect on the organization of the retina.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Mitchell D. E. Environmental modification of the visual cortex and the neural basis of learning and memory. Nature. 1973 Feb 16;241(5390):467–468. doi: 10.1038/241467a0. [DOI] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Cats reared in stroboscopic illumination: effects on receptive fields in visual cortex. Proc Natl Acad Sci U S A. 1973 May;70(5):1353–1354. doi: 10.1073/pnas.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews P. B., Wiesel T. N. Consequences of monocular deprivation on visual behaviour in kittens. J Physiol. 1970 Feb;206(2):437–455. doi: 10.1113/jphysiol.1970.sp009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifková E. Effect of light and visual deprivation on the retina. Exp Neurol. 1972 Jun;35(3):450–457. doi: 10.1016/0014-4886(72)90115-x. [DOI] [PubMed] [Google Scholar]
- Fifková E. Effect of visual deprivation and light on synapses of the inner plexiform layer. Exp Neurol. 1972 Jun;35(3):458–469. doi: 10.1016/0014-4886(72)90116-1. [DOI] [PubMed] [Google Scholar]
- Freeman R. D., Mitchell D. E., Millodot M. A neural effect of partial visual deprivation in humans. Science. 1972 Mar 24;175(4028):1384–1386. doi: 10.1126/science.175.4028.1384. [DOI] [PubMed] [Google Scholar]
- Grobstein P., Chow K. L., Spear P. D., Mathers L. H. Development of rabbit visual cortex: late appearance of a class of receptive fields. Science. 1973 Jun 15;180(4091):1185–1187. doi: 10.1126/science.180.4091.1185. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972 Jan;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- Guillery R. W., Stelzner D. J. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970 Aug;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki D. I., Pollack J. G. Depression of the late receptor potential and the ERG by light deprivation in cats. Vision Res. 1972 May;12(5):835–842. doi: 10.1016/0042-6989(72)90009-0. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970 May 15;168(3933):869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V. Visual perception in cats after environmental surgery. Exp Brain Res. 1972;15(4):405–423. doi: 10.1007/BF00234126. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. A schematic eye for the rabbit. Vision Res. 1972 Jan;12(1):123–138. doi: 10.1016/0042-6989(72)90143-5. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles F. A. Centrifugal control of the avian retina. I. Receptive field properties of retinal ganglion cells. Brain Res. 1972 Dec 24;48:65–92. doi: 10.1016/0006-8993(72)90171-0. [DOI] [PubMed] [Google Scholar]
- Mize R. R., Murphy E. H. Selective visual experience fails to modify receptive field properties of rabbit striate cortex neurons. Science. 1973 Apr 20;180(4083):320–323. doi: 10.1126/science.180.4083.320. [DOI] [PubMed] [Google Scholar]
- NOELL W. K. Differentiation, metabolic organization, and viability of the visual cell. AMA Arch Ophthalmol. 1958 Oct;60(4 Pt 2):702–733. doi: 10.1001/archopht.1958.00940080722016. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D. The importance of early visual experience for neurons of the developing geniculostriate system. Invest Ophthalmol. 1972 May;11(5):386–394. [PubMed] [Google Scholar]
- RIESEN A. H., KURKE M. I., MELLINGER J. C. Interocular transfer of habits learned monocularly in visually naive and visually experienced cats. J Comp Physiol Psychol. 1953 Jun;46(3):166–172. doi: 10.1037/h0057003. [DOI] [PubMed] [Google Scholar]
- Reuter J. H., Legein C. P., van der Mark F., van Hof M. W. The electroretinogram in normal and light-deprived rabbits. Doc Ophthalmol. 1971 Sep 12;30:349–361. doi: 10.1007/BF00142531. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Stone J. Physiological normality of the retinal in visually deprived cats. Brain Res. 1973 Sep 28;60(1):224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Sosula L., Glow P. H. Increase in number of synapses in the inner plexiform layer of light deprived rat retinae: quantitative electron microscopy. J Comp Neurol. 1971 Apr;141(4):427–451. doi: 10.1002/cne.901410403. [DOI] [PubMed] [Google Scholar]
- Spear P. D., Chow K. L., Masland R. H., Murphy E. H. Ontogenesis of receptive field characteristics of superior colliculus neurons in the rabbit. Brain Res. 1972 Oct 13;45(1):67–86. doi: 10.1016/0006-8993(72)90216-8. [DOI] [PubMed] [Google Scholar]
- WEISKRANTZ L. Sensory deprivation and the cat's optic nervous system. Nature. 1958 Apr 12;181(4615):1047–1050. doi: 10.1038/1811047b0. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


