Abstract
1. Methods for measuring the release of 45Ca from isolated urinary bladders of toads (Bufo marinus) pre-loaded with this isotope have been devised. One method allowed separate collection from the mucosal and serosal surfaces of the bladders.
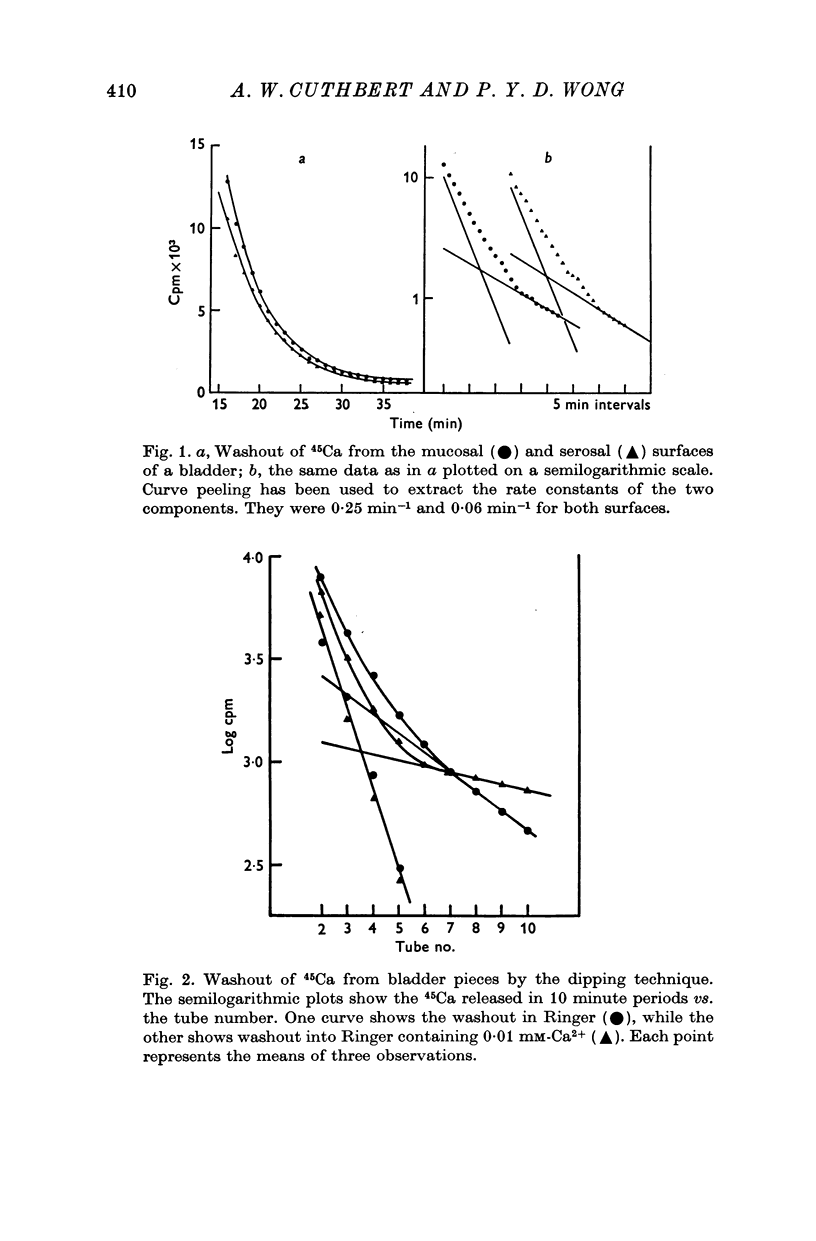
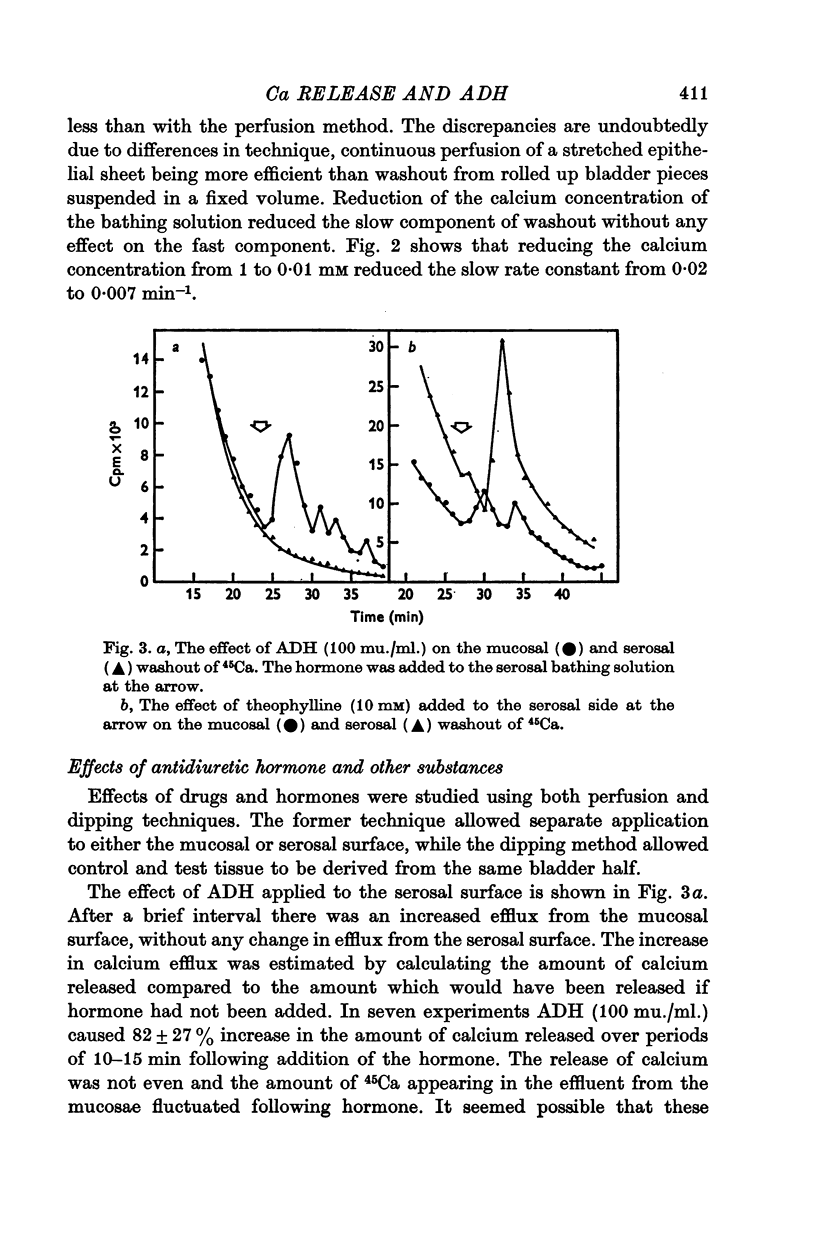
2. Reducing the ambient calcium concentration reduced the rate of 45Ca efflux suggesting that efflux of radiolabel represents calcium exchange.
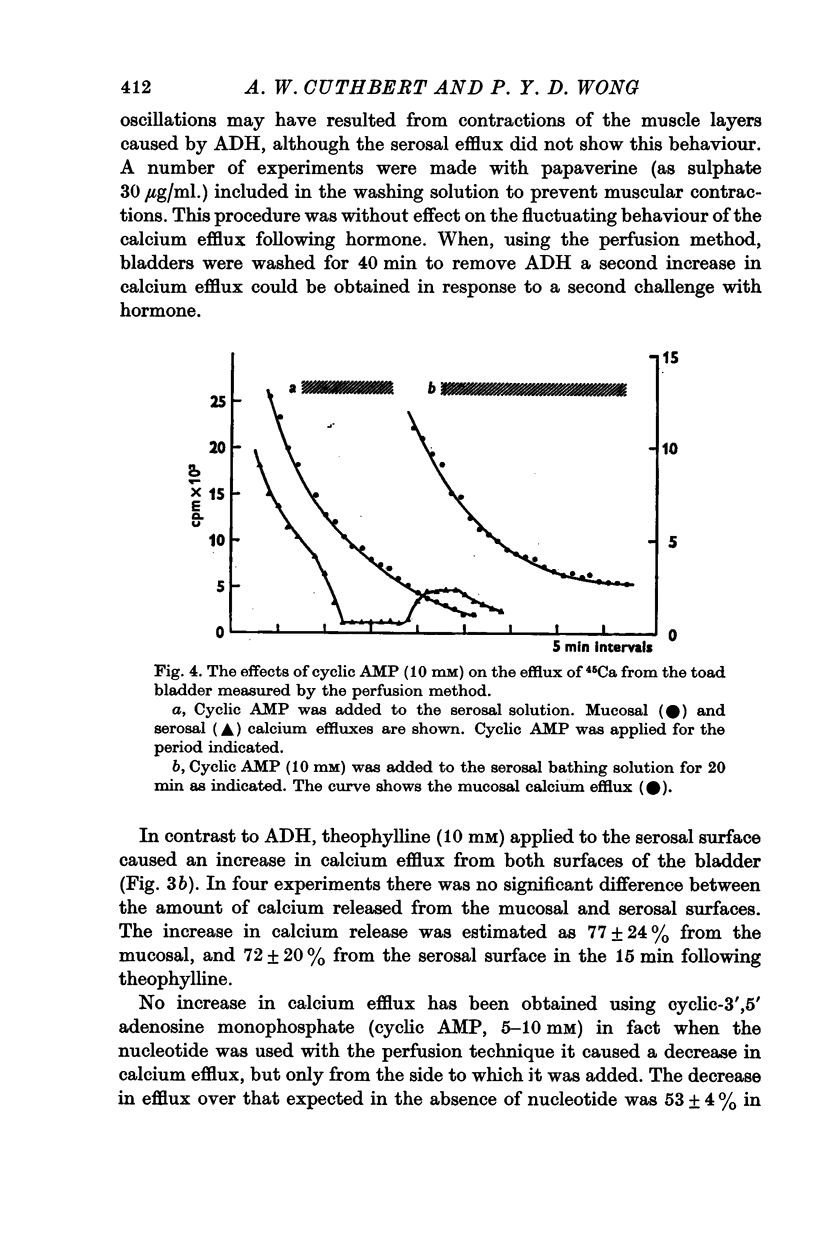
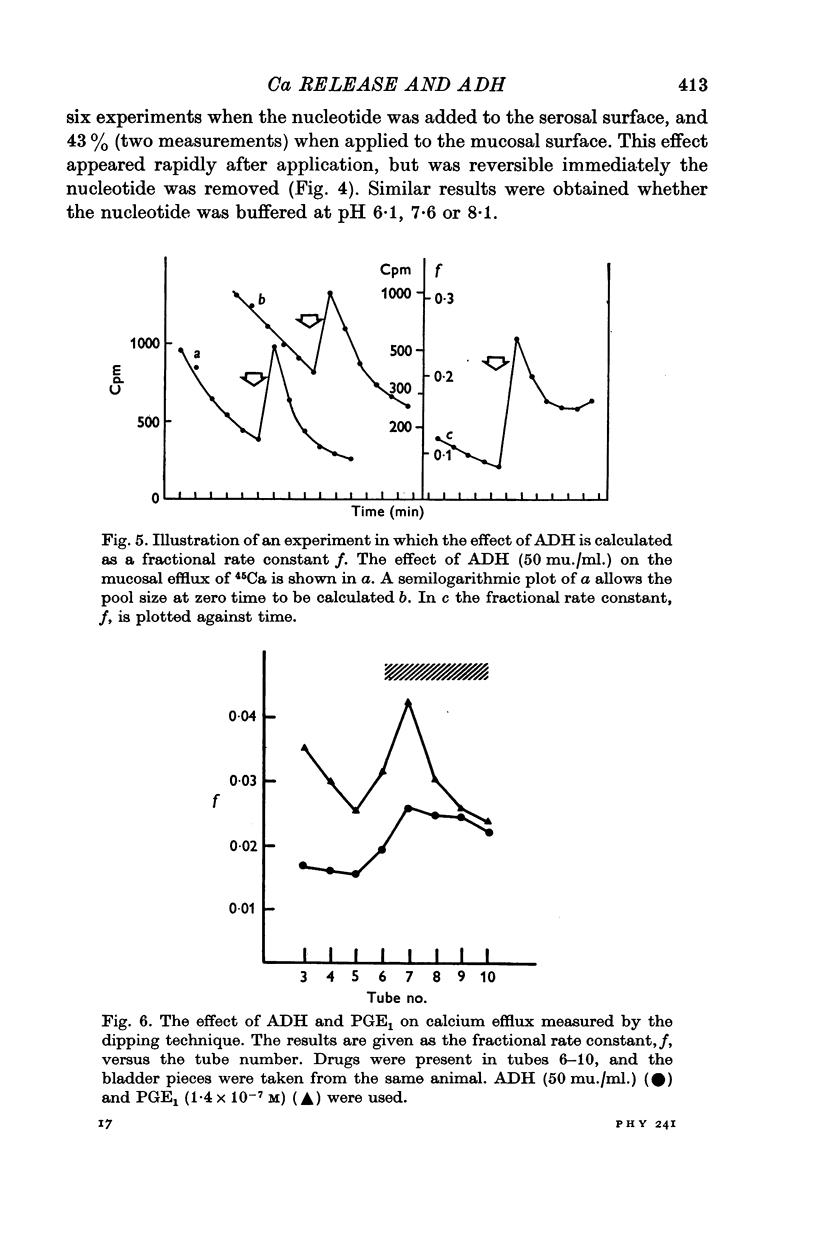
3. Antidiuretic hormone, theophylline and prostaglandin E1 all increased calcium efflux, while lanthanum and amphotericin were without effect. Cyclic AMP caused only an inhibition of calcium release.
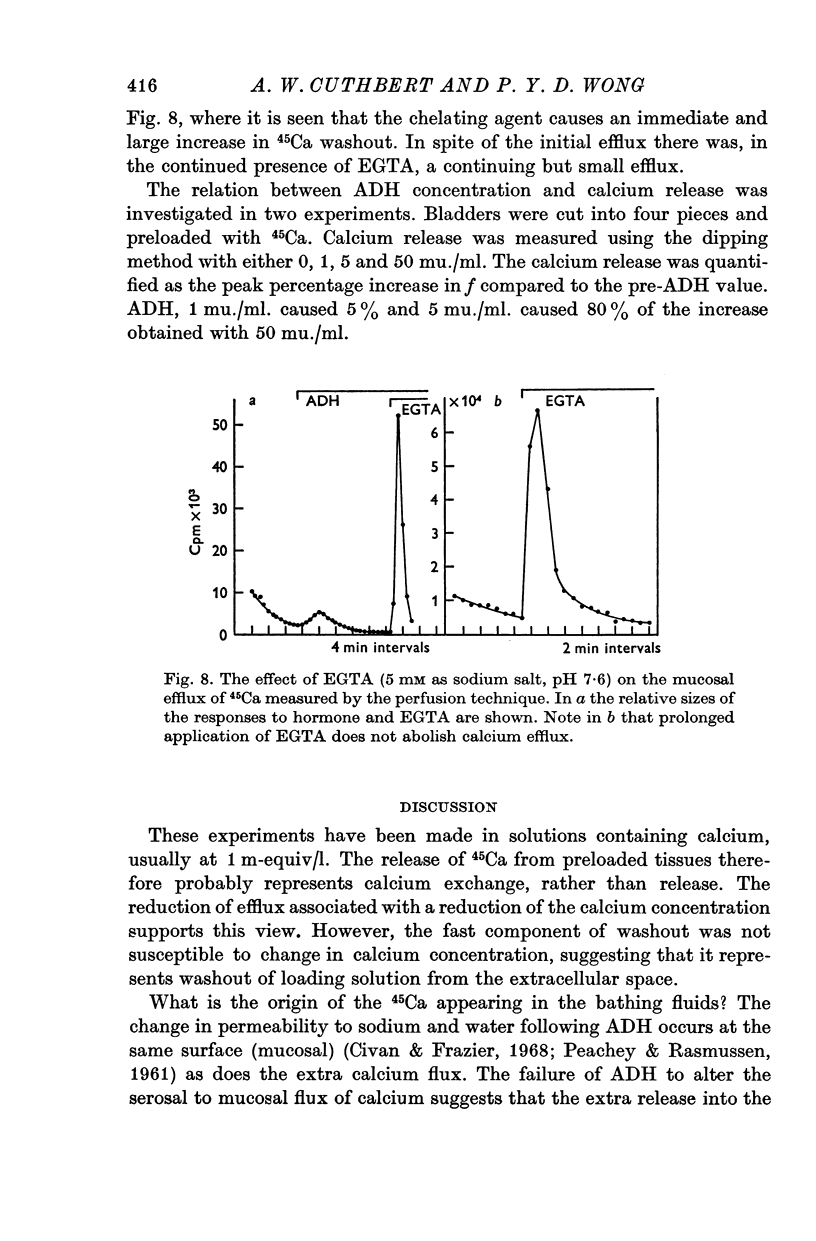
4. The increase in 45Ca efflux due to antidiuretic hormone came exclusively from the mucosal side. Experiments with EGTA suggest that the calcium entering the mucosal solution arises mainly from superficial sites in the mucosal membrane.
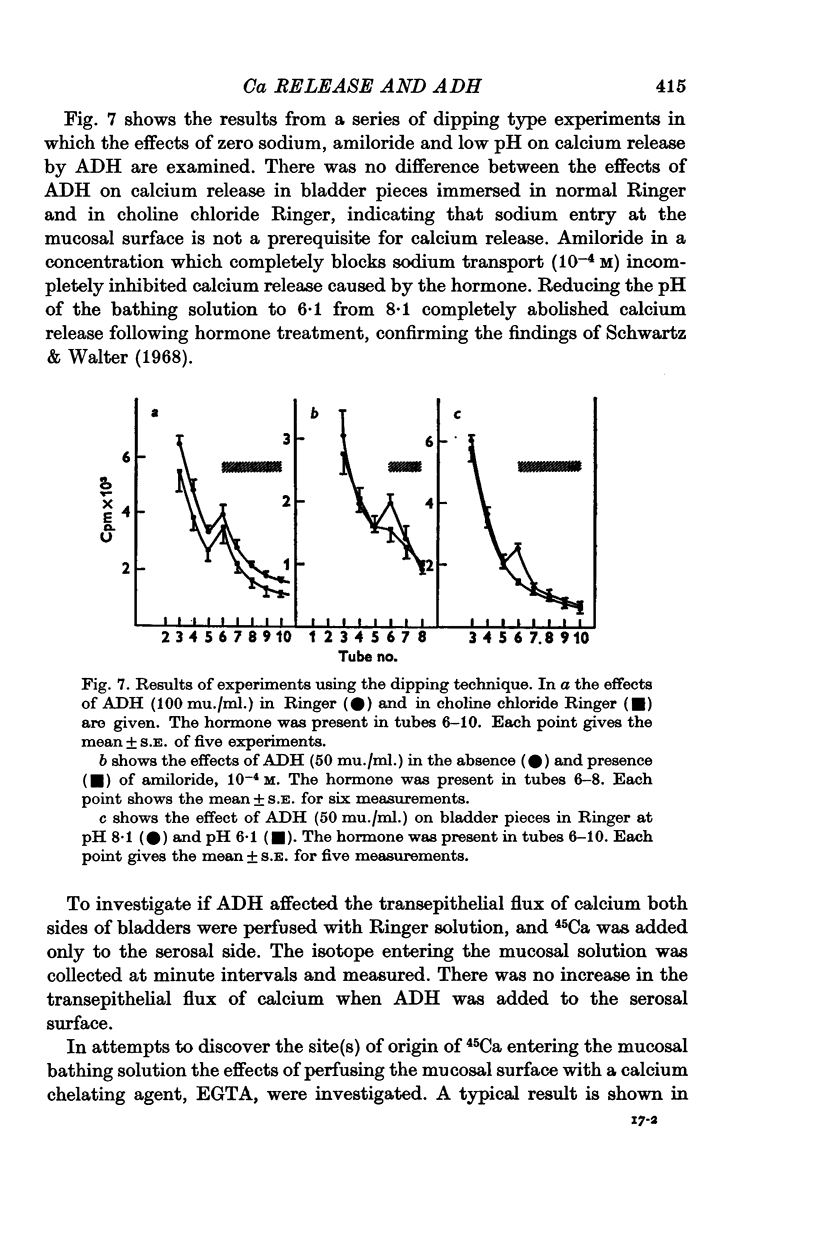
5. The release of 45Ca by hormone was not influenced by removal of sodium from the bathing solution. Low pH and amiloride reduced or abolished calcium release to hormone.
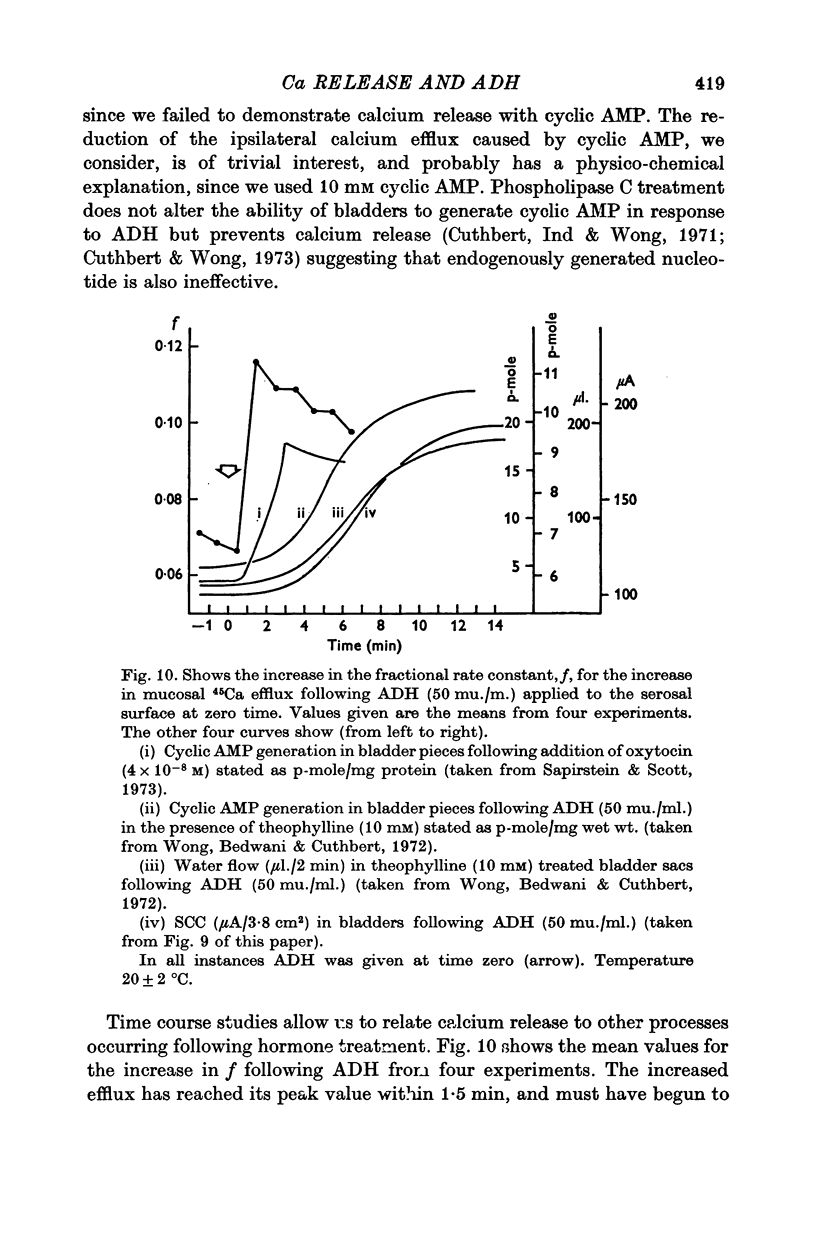
6. The time course of calcium release from the mucosal surface due to hormone was rapid (commencing between 0·5 and 1·5 min after hormone application). Thus calcium release precedes the increase in sodium transport and hydro-osmotic flow following hormone, and appears to be at least as rapid as cyclic AMP generation in the tissue.
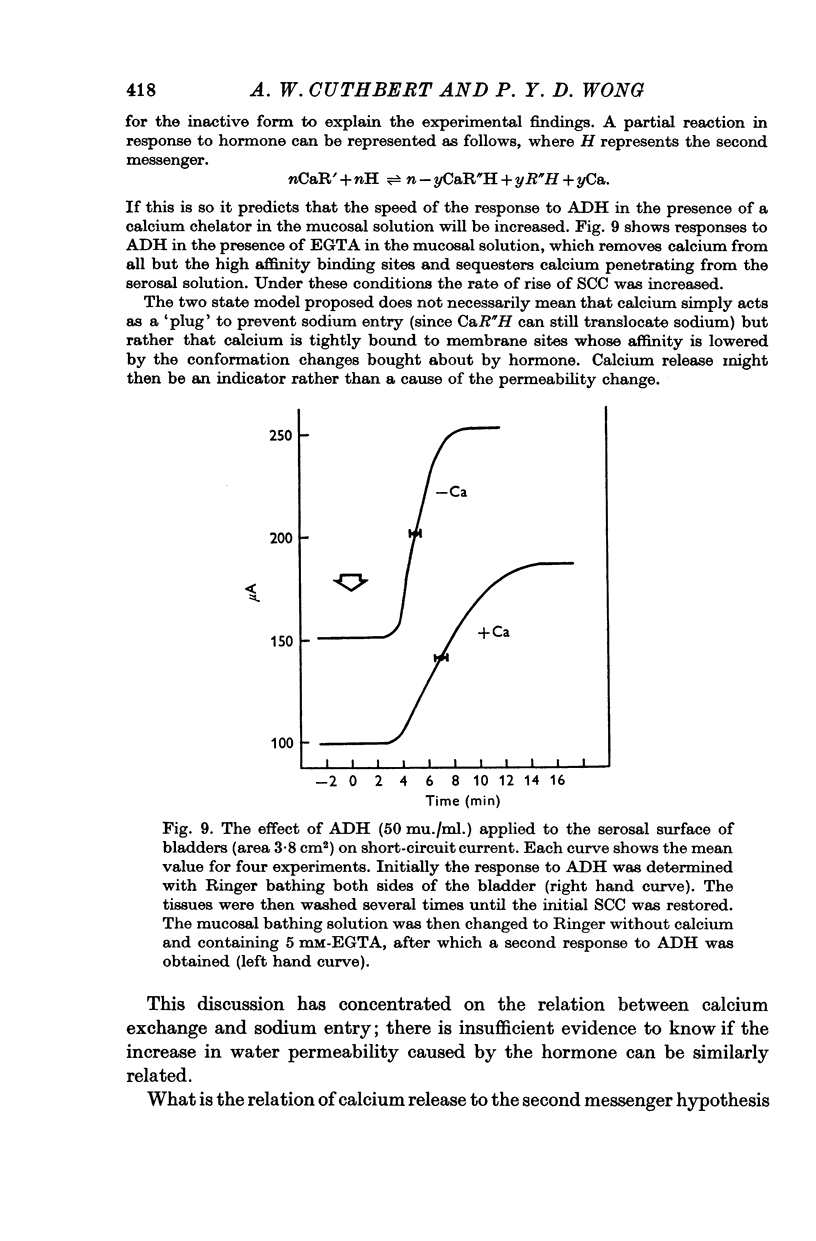
7. The relationship between calcium release or exchange and the permeability changes in the bladder to water and to sodium, following hormone, are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P. J. Action of amphotericin B on the toad bladder: evidence for sodium transport along two pathways. J Physiol. 1968 Jun;196(3):703–711. doi: 10.1113/jphysiol.1968.sp008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Frazier H. S. The site of the stimulatory action of vasopressin on sodium transport in toad bladder. J Gen Physiol. 1968 May;51(5):589–605. doi: 10.1085/jgp.51.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W. An upper limit to the number of sodium channels in frog skin epithelium. J Physiol. 1973 Feb;228(3):681–692. doi: 10.1113/jphysiol.1973.sp010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Ind P. W., Wong P. Y. Increased concentrations of cyclic 3',5'-adenosine monophosphate without a physiological response after antidiuretic hormone. Br J Pharmacol. 1971 Dec;43(4):881–884. doi: 10.1111/j.1476-5381.1971.tb07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E. Independent action of antidiuretic hormone, theophylline and cyclic 3',5'-adenosine monophosphate on cell membrane permeability in frog skin. J Physiol. 1968 Dec;199(3):593–612. doi: 10.1113/jphysiol.1968.sp008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E., Prince W. T. The effects of anions on sodium transport. Br J Pharmacol. 1969 May;36(1):97–106. doi: 10.1111/j.1476-5381.1969.tb08307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter M. E. Modifications of the responses to antidiuretic hormone by hydrolytic enzymes. J Pharm Pharmacol. 1971 Apr;23(4):262–269. doi: 10.1111/j.2042-7158.1971.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. Amiloride, Ca 2+ and oxygen consumption of the urinary bladders of toads. Biochim Biophys Acta. 1971 Aug 13;241(2):713–715. doi: 10.1016/0005-2736(71)90074-5. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. Effect of phospholipase C on calcium release from epithelia treated with antidiuretic hormone. Br J Pharmacol. 1973 Apr;47(4):778–781. doi: 10.1111/j.1476-5381.1973.tb08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. The effect of metal ions and antidiuretic hormone on oxygen consumption in toad bladder. J Physiol. 1971 Dec;219(1):39–56. doi: 10.1113/jphysiol.1971.sp009648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. The role of calcium ions in the interaction of amiloride with membrane receptors. Mol Pharmacol. 1972 Mar;8(2):222–229. [PubMed] [Google Scholar]
- Erlij D. Salt transport across isolated frog skin. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):153–161. doi: 10.1098/rstb.1971.0086. [DOI] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. R., Smith M. W. Direct measurement of sodium uptake by toad bladder mucosal cells. J Endocrinol. 1972 Oct;55(1):195–201. doi: 10.1677/joe.0.0550195. [DOI] [PubMed] [Google Scholar]
- Finn A. L. The kinetics of sodium transport in the toad bladder. II. Dual effects of vasopressin. J Gen Physiol. 1971 Mar;57(3):349–362. doi: 10.1085/jgp.57.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyassy P. F., Edelman I. S. Hydrogen-ion dependence of the antidiuretic action of vasopressin, oxytocin and deaminooxytocin. Biochim Biophys Acta. 1965 May 25;102(1):185–197. doi: 10.1016/0926-6585(65)90212-8. [DOI] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACHEY L. D., RASMUSSEN H. Structure of the toad's urinary bladder as related to its physiology. J Biophys Biochem Cytol. 1961 Aug;10:529–553. doi: 10.1083/jcb.10.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN H., SCHWARTZ I. L., YOUNG R., MARC-AURELE J. STRUCTURAL REQUIREMENTS FOR THE ACTION OF NEUROHYPOPHYSEAL HORMONES UPON THE ISOLATED AMPHIBIAN URINARY BLADDER. J Gen Physiol. 1963 Jul;46:1171–1189. doi: 10.1085/jgp.46.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Scott W. N. Cyclic AMP and sodium transport. Quantitative and temporal relationships in toad urinary bladder. J Clin Invest. 1973 Sep;52(9):2379–2382. doi: 10.1172/JCI107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Y., Bedwani J. R., Cuthbert A. W. Hormone action and the levels of cyclic AMP and prostaglandins in the toad bladder. Nat New Biol. 1972 Jul 5;238(79):27–31. doi: 10.1038/newbio238027a0. [DOI] [PubMed] [Google Scholar]


