Abstract
1. Six healthy males performed sustained contractions with different tensions related to their maximal voluntary contraction (MVC). The isometric exercise consisted of efforts to extend the knee when flexed at an angle of 90°.
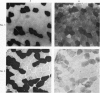
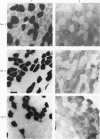
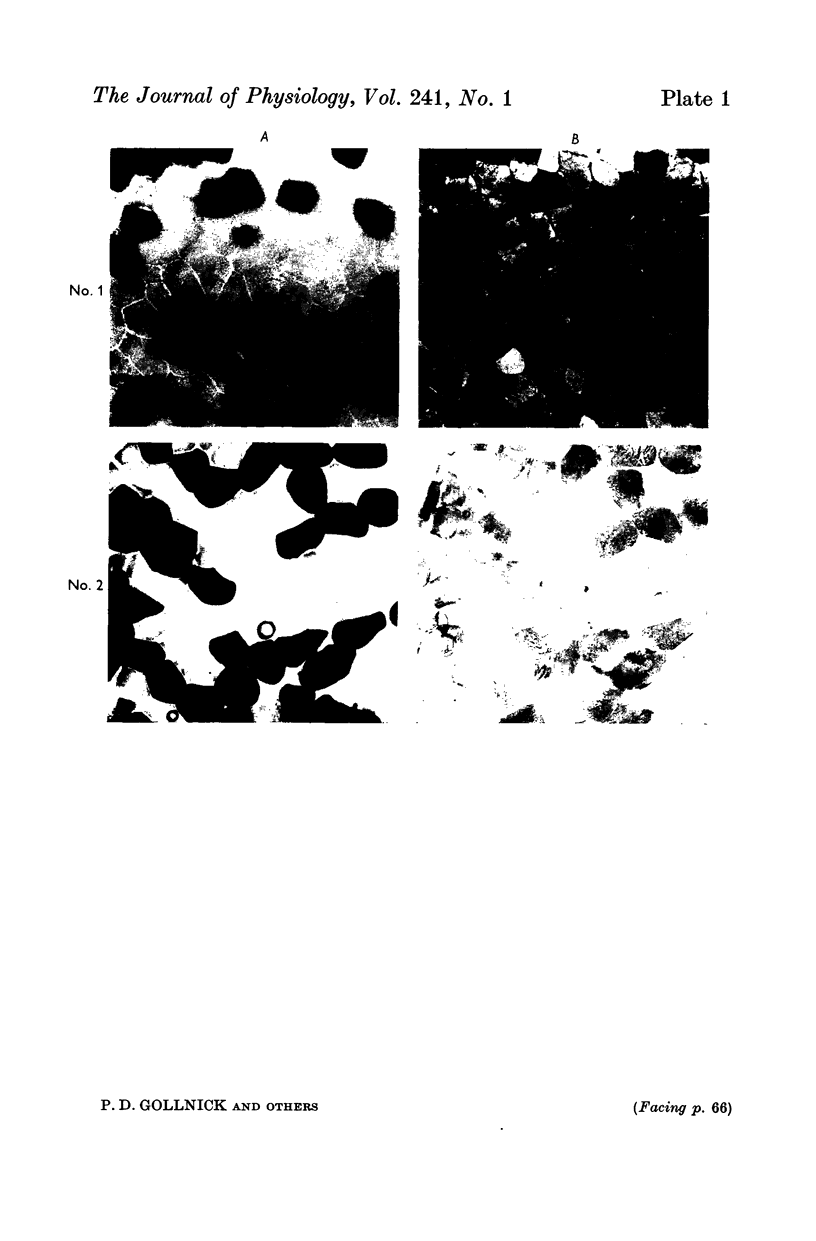
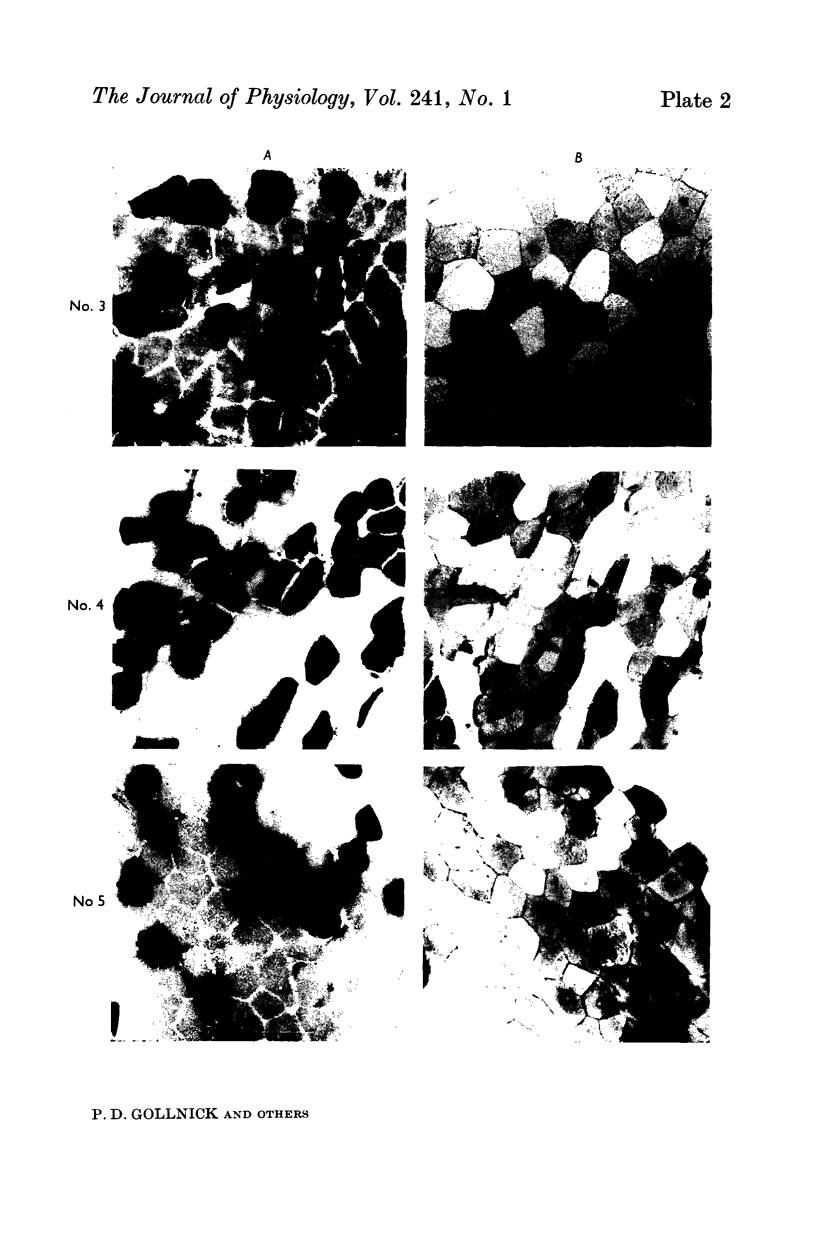
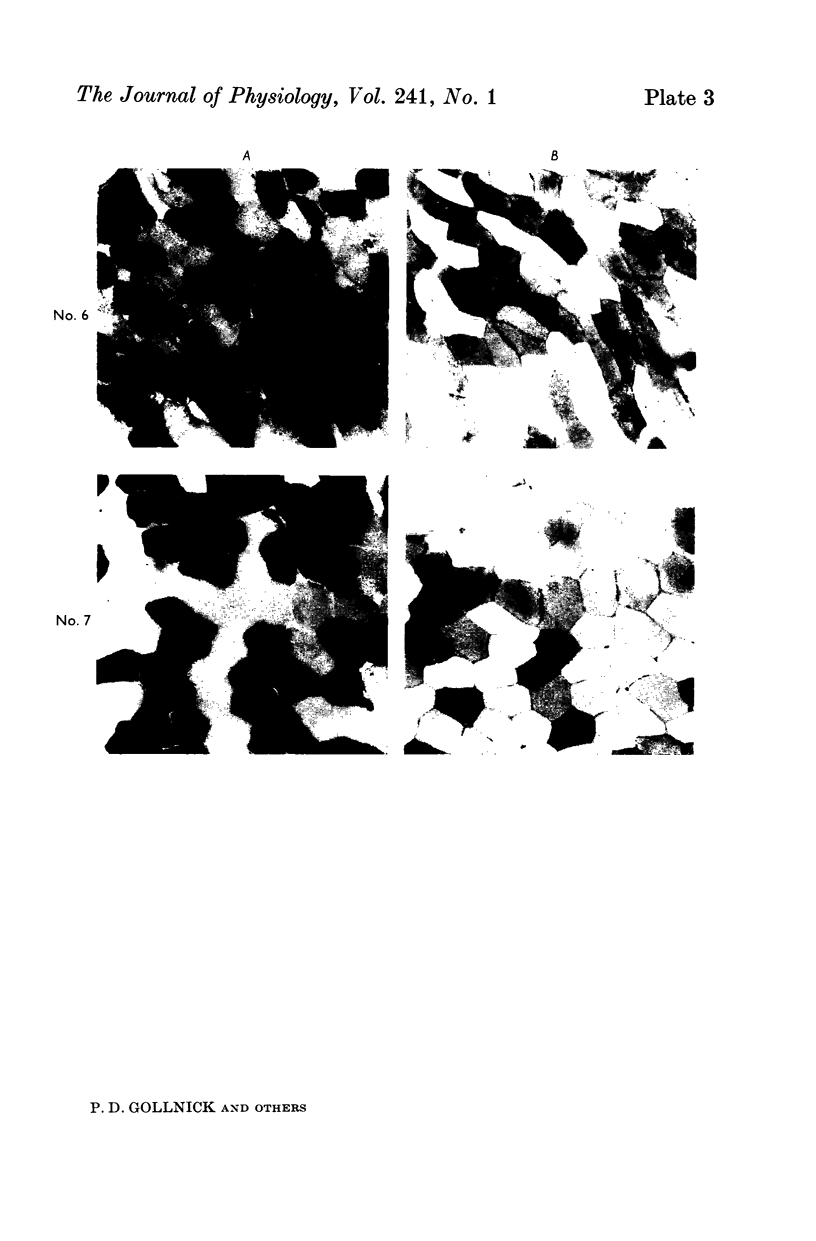
2. Biopsy samples were taken from the lateral portion of M. quadriceps femoris before and after different periods (6-45 min) during a series of sustained contractions. Total glycogen content was determined on each muscle sample. In order to evaluate whether the glycogen depletion occurred preferentially in slow twitch (ST) or fast twitch (FT) fibres, serial sections of the muscle samples were stained for myofibrillar ATPase and glycogen (PAS reaction).
3. In all experiments a selective glycogen depletion was observed. At low tensions, the ST fibres and at higher tensions the FT fibres became glycogen depleted. The critical tension at which this conversion in glycogen depletion from ST to FT fibres took place was 20% MVC.
4. It is concluded that at sustained contractions of less than 20% MVC there is a major reliance upon ST fibres and above that level a primary dependence upon FT fibres. It is further suggested that restriction of blood flow and thus availability of oxygen at forces higher than 20% MVC may be the explanation for the present findings.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcroft H., Millen J. L. The blood flow through muscle during sustained contraction. J Physiol. 1939 Nov 14;97(1):17–31. doi: 10.1113/jphysiol.1939.sp003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol. 1968 Jun;196(3):631–654. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W. K. Selective and nonselective susceptibility of muscle fiber types. A new approach to human neuromuscular diseases. Arch Neurol. 1970 Feb;22(2):97–117. doi: 10.1001/archneur.1970.00480200003001. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saubert C. W., 4th, Piehl K., Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972 Sep;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974 Aug;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saubert C. W., 4th, Armstrong R. B., Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972 Oct;33(4):421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- Grimby L., Hannerz J. Recruitment order of motor units on voluntary contraction: changes induced by proprioceptive afferent activity. J Neurol Neurosurg Psychiatry. 1968 Dec;31(6):565–573. doi: 10.1136/jnnp.31.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS P. W., LIND A. R. The blood flow through active and inactive muscles of the forearm during sustained hand-grip contractions. J Physiol. 1963 Apr;166:120–135. doi: 10.1113/jphysiol.1963.sp007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Ollander B. Muscle metabolites with exhaustive static exercise of different duration. Acta Physiol Scand. 1972 Nov;86(3):309–314. doi: 10.1111/j.1748-1716.1972.tb05337.x. [DOI] [PubMed] [Google Scholar]
- MONOD H., SCHERRER J. Capacité de travail statique d'un groupe musculaire synergique chez l'homme. C R Seances Soc Biol Fil. 1957;151(7):1358–1362. [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Piehl K. Time course for refilling of glycogen stores in human muscle fibres following exercise-induced glycogen depletion. Acta Physiol Scand. 1974 Feb;90(2):297–302. doi: 10.1111/j.1748-1716.1974.tb05592.x. [DOI] [PubMed] [Google Scholar]
- ROHMERT W. [Determination of the recovery pause for static work of man]. Int Z Angew Physiol. 1960;18:123–164. [PubMed] [Google Scholar]