Abstract
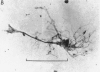
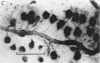
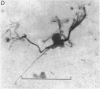
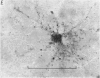
1. The dry mass and nucleic acid content of freshly isolated neuroglial cells and of their nucleoli were measured by interference microscopy and ultraviolet absorption microspectrography. The incorporation of tritiated nucleosides and of an amino acid was followed autoradiographically.
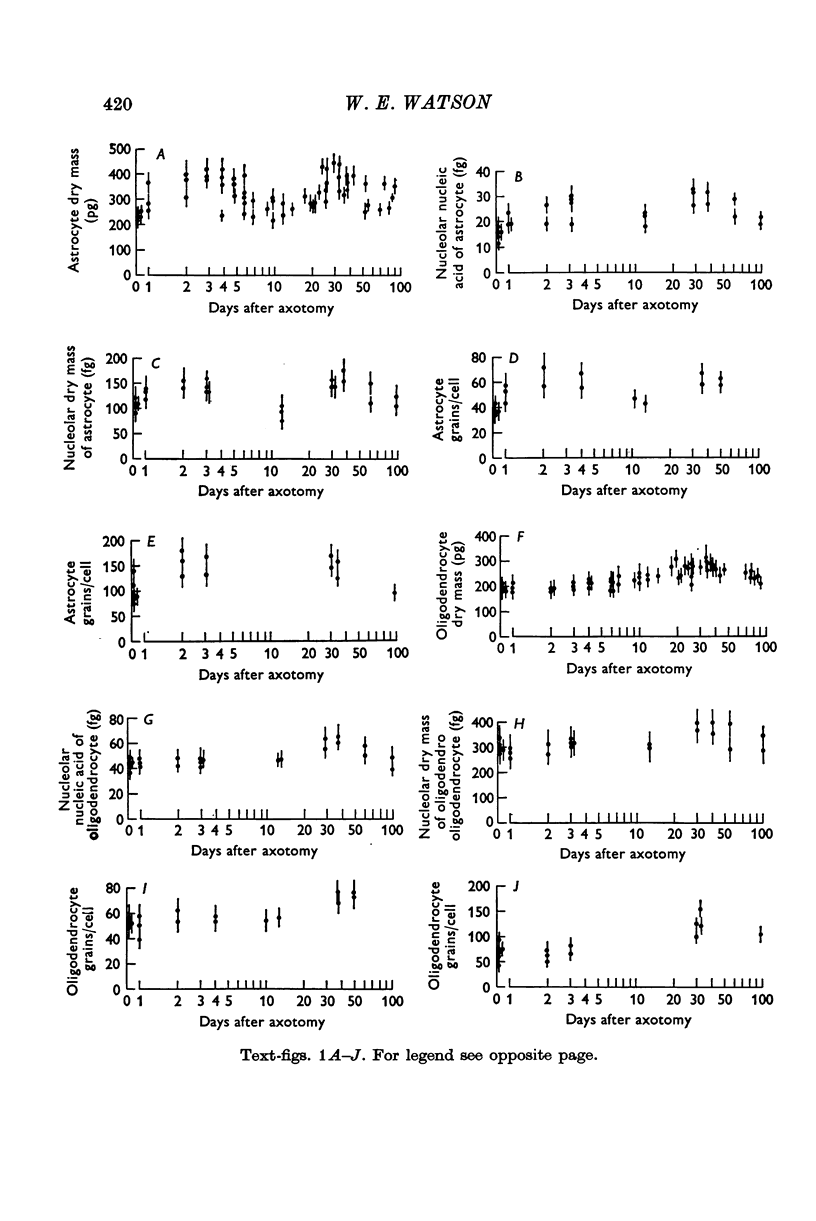
2. After hypoglossal axotomy in the adult rat hypertrophy of astrocytes of the hypoglossal nucleus occurred in a biphasic manner. The first phase lasted from days 1-10 and was accompanied by a small degree of astrocytic hyperplasia, and the second from days 20-80. Hypertrophy of oligodendrocytes accompanied the second phase of the astrocytic response.
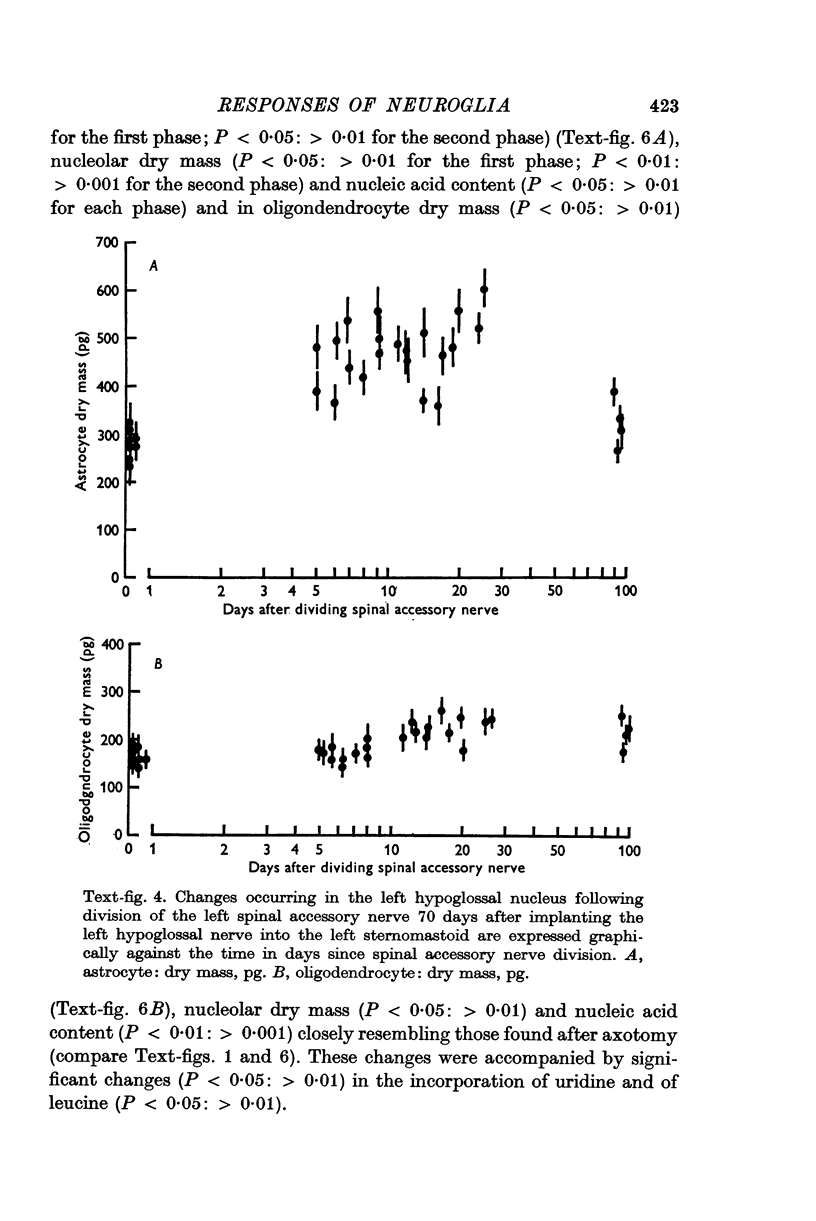
3. When severed axons failed to reinnervate denervated muscle, the second phase of the astrocytic response was markedly reduced and the hypertrophy of oligodendrocytes did not occur.
4. If the severed axons re-innervated denervated muscle after a controlled delay, the second phase of the astrocytic response and the oligodendroglial hypertrophy was also delayed.
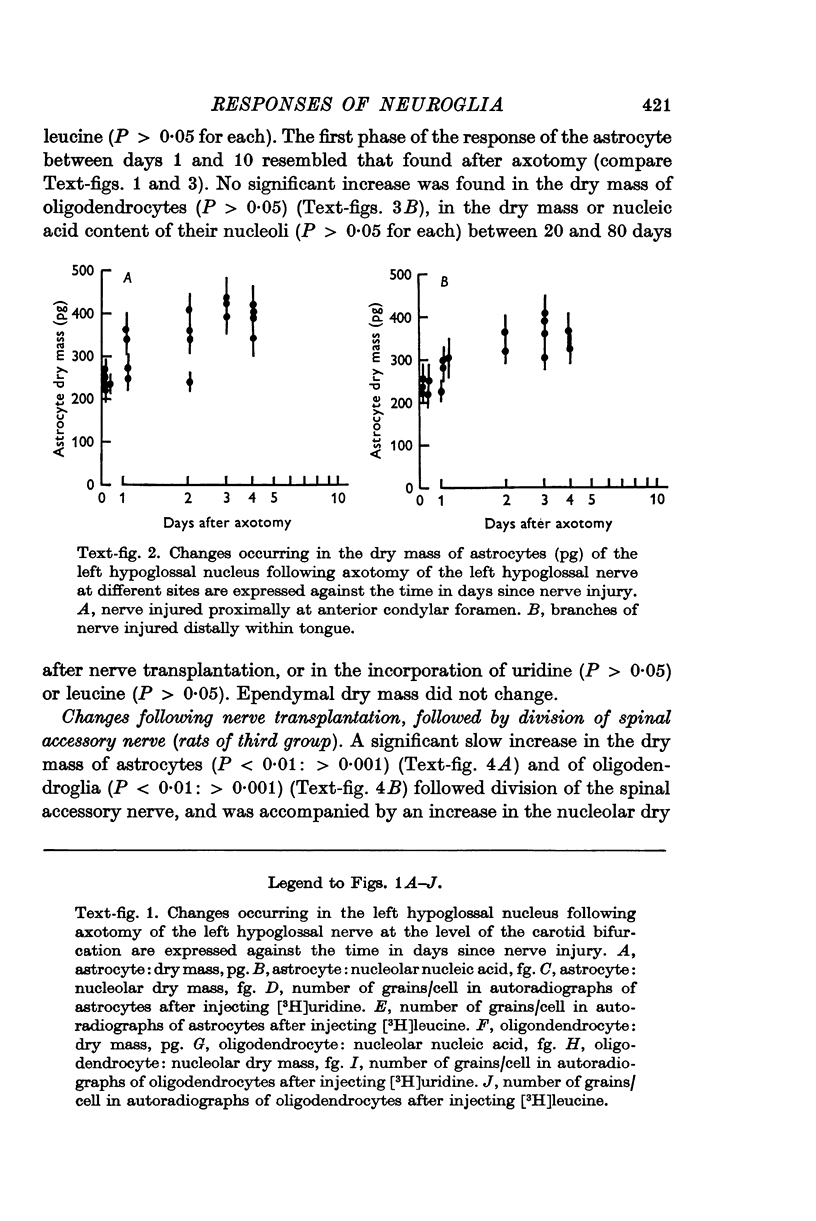
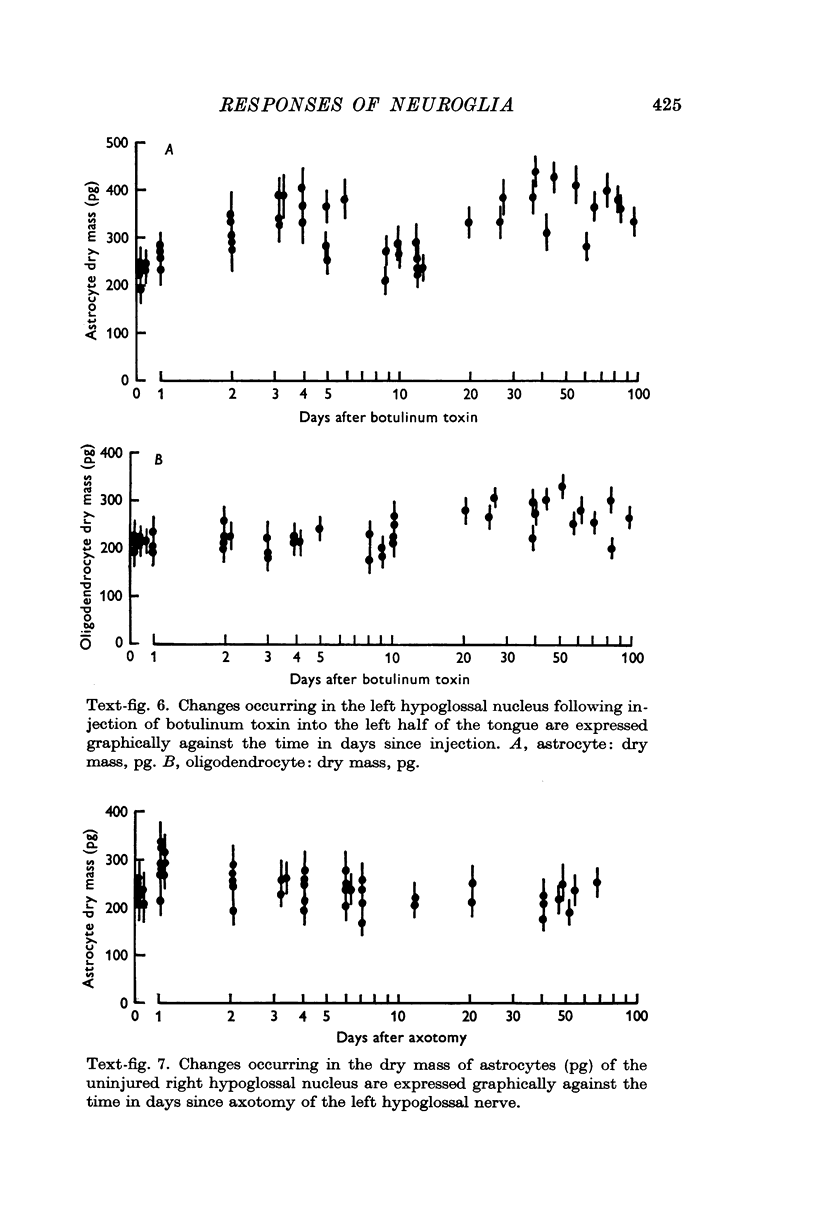
5. Injection of botulinum toxin into the tongue caused changes in astrocytes and oligondendrocytes closely resembling those found after axotomy.
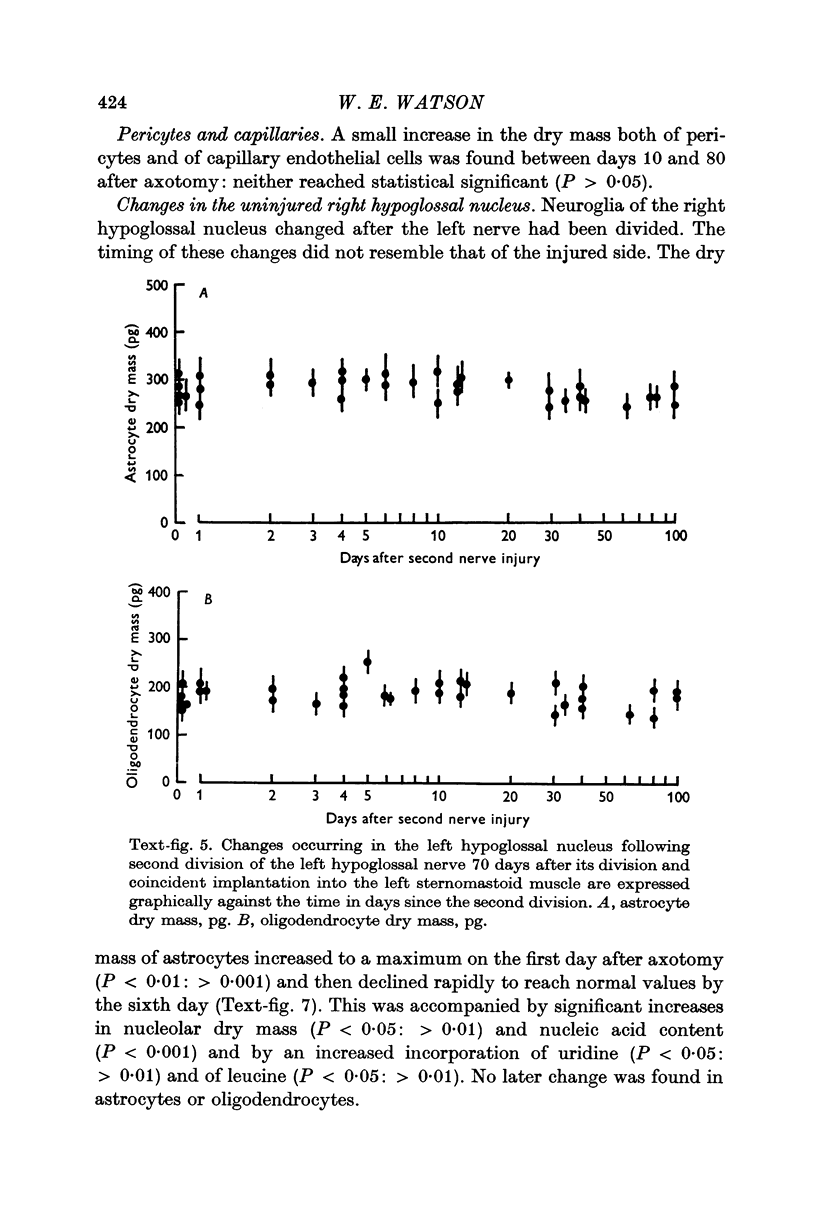
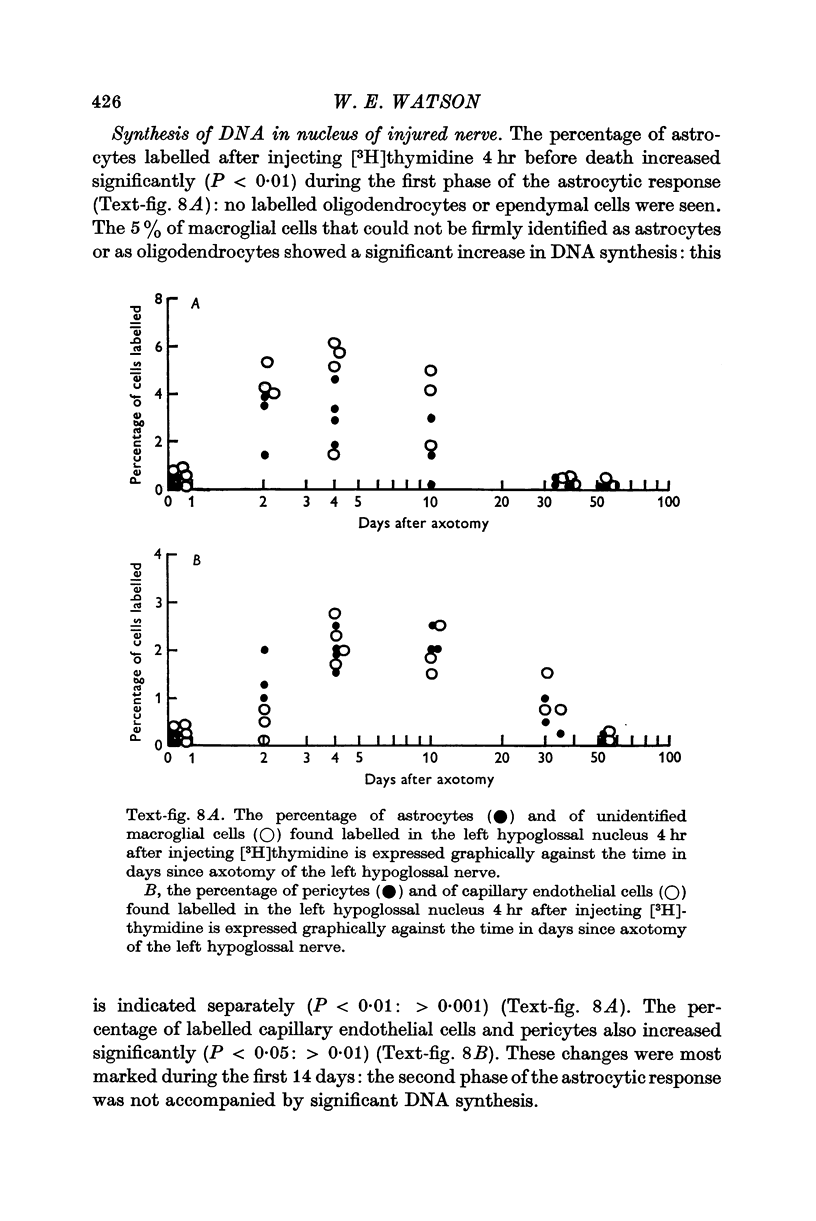
6. Transient astrocytic hypertrophy occurred in the uninjured right hypoglossal nucleus, and had a different time course to the changes occurring on the injured side.
7. The results are discussed in relation to changes in the metabolism and to alterations in the dendritic fields of injured neurones, previously measured in these circumstances.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. K., Jr, Smothermon R. D. Leucocytic infiltration into the hypoglossal nucleus following injury to the hypoglossal nerve. Anat Rec. 1970 Jan;166(1):99–115. doi: 10.1002/ar.1091660108. [DOI] [PubMed] [Google Scholar]
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K., Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A. Anatomical studies of cerebellar fibre connections with special reference to problems of functional localization. Prog Brain Res. 1967;25:135–173. doi: 10.1016/S0079-6123(08)60964-4. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- CAMMERMEYER J. Astroglial changes during retrograde atrophy of nucleus facialis in mice. J Comp Neurol. 1955 Feb;102(1):133–150. doi: 10.1002/cne.901020107. [DOI] [PubMed] [Google Scholar]
- CARVALHO A. P., SANUI H., PACE N. CALCIUM AND MAGNESIUM BINDING PROPERTIES OF CELL MEMBRANE MATERIALS. J Cell Physiol. 1963 Dec;62:311–317. doi: 10.1002/jcp.1030620311. [DOI] [PubMed] [Google Scholar]
- CERF J. A., CHACKO L. W. Retrograde reaction in motoneuron dendrites following ventral root section in the frog. J Comp Neurol. 1958 Apr;109(2):205–219. doi: 10.1002/cne.901090205. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The effect of diffusional barriers upon the pharmacology of cells within the central nervous system. J Physiol. 1958 May 28;141(3):446–463. doi: 10.1113/jphysiol.1958.sp005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J. B. The proliferation of astrocytes around a needle wound in the rat brain. J Anat. 1970 May;106(Pt 3):471–487. [PMC free article] [PubMed] [Google Scholar]
- Croft C. B., Tarin D. Ultrastructural studies of wound healing in mouse skin. I. Epithelial behaviour. J Anat. 1970 Jan;106(Pt 1):63–77. [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES D. M., FATT P. Pharmacological investigations on a central synapse operated by acetylcholine. J Physiol. 1956 Jan 27;131(1):154–169. doi: 10.1113/jphysiol.1956.sp005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin I. Mesenchymal tissues of the nervous system. The indigenous origin of brain macrophages in hypoxic states and in multiple sclerosis. J Neuropathol Exp Neurol. 1969 Jan;28(1):6–24. doi: 10.1097/00005072-196901000-00002. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Johnstone M. A. Responses of thymidine labeling of nuclei in gray matter and nerve following sciatic transection. Acta Neuropathol. 1967 Jan 2;7(3):218–231. doi: 10.1007/BF00686373. [DOI] [PubMed] [Google Scholar]
- GARBER B. Inhibition by glucosamine of aggregation of dissociated embryonic cells. Dev Biol. 1963 Mar;6:630–641. doi: 10.1016/0012-1606(63)90147-7. [DOI] [PubMed] [Google Scholar]
- Grossman R. G., Whiteside L., Hampton T. L. The time course of evoked depolarization of cortical glial cells. Brain Res. 1969 Jul;14(2):401–415. doi: 10.1016/0006-8993(69)90118-8. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Uretsky N. J. Regional effects of 6-hydroxydopamine on catecholamine containing neurones in rat brain and spinal cord. Brain Res. 1970 Dec 1;24(2):364–367. doi: 10.1016/0006-8993(70)90121-6. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., POTTER D. D. GLIA IN THE LEECH CENTRAL NERVOUS SYSTEM: PHYSIOLOGICAL PROPERTIES AND NEURON-GLIA RELATIONSHIP. J Neurophysiol. 1964 Mar;27:290–320. doi: 10.1152/jn.1964.27.2.290. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. How do materials exchange between blood and nerve cells in the brain? Perspect Biol Med. 1965 Autumn;9(1):69–76. doi: 10.1353/pbm.1965.0017. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dowling J. E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970 May;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- OSTERBERG K. A., WATTENBERG L. W. Inductive factors in gliosis. Proc Soc Exp Biol Med. 1962 Nov;111:452–455. doi: 10.3181/00379727-111-27822. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Peters A., Palay S. L. The morphology of laminae A and A1 of the dorsal nucleus of the lateral geniculate body of the cat. J Anat. 1966 Jul;100(Pt 3):451–486. [PMC free article] [PubMed] [Google Scholar]
- Roessmann U., Friede R. L. Entry of labeled monocytic cells into the central nervous system. Acta Neuropathol. 1968 Jun 7;10(4):359–362. doi: 10.1007/BF00690711. [DOI] [PubMed] [Google Scholar]
- Ross R., Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J Cell Biol. 1968 Oct;39(1):152–168. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff R. P., Vaughn J. E. An autoradiographic study of cellular proliferation in degenerating rat optic nerve. J Comp Neurol. 1971 Feb;141(2):133–155. doi: 10.1002/cne.901410202. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. A simple method for obtaining autoradiographs of single isolated neuroglial cells. J Physiol. 1972 Apr;222(2):119P–120P. [PMC free article] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971 Sep 24;233(5317):273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Tarin D., Croft C. B. Ultrastructural studies of wound healing in mouse skin. II. Dermo-epidermal interrelationships. J Anat. 1970 Jan;106(Pt 1):79–91. [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg M. C., Pollen D. A. Neuroglia: biophysical properties and physiologic function. Science. 1970 Feb 27;167(3922):1248–1252. doi: 10.1126/science.167.3922.1248. [DOI] [PubMed] [Google Scholar]
- VRBA R., FOLBERGR J., KANTUREK V. On the mechanism of ammonia formation in guinea pig brain slices. J Neurochem. 1958;2(2-3):187–196. doi: 10.1111/j.1471-4159.1958.tb12363.x. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E. An electron microscopic analysis of gliogenesis in rat optic nerves. Z Zellforsch Mikrosk Anat. 1969;94(3):293–324. doi: 10.1007/BF00319179. [DOI] [PubMed] [Google Scholar]
- Watson W. E. A quantitative study of some neuroglial responses to neuronal stimulation. J Physiol. 1972 Sep;225(2):54P–56P. [PubMed] [Google Scholar]
- Watson W. E. Alteration of the adherence of glia to neurons following nerve injury. J Neurochem. 1966 Jun;13(6):536–537. doi: 10.1111/j.1471-4159.1966.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Quantitative observations upon acetylcholine hydrolase activity of nerve cells after axotomy. J Neurochem. 1966 Dec;13(12):1549–1550. doi: 10.1111/j.1471-4159.1966.tb04322.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. Some metabolic responses of axotomized neurones to contact between their axons and denervated muscle. J Physiol. 1970 Sep;210(2):321–343. doi: 10.1113/jphysiol.1970.sp009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Some quantitative observations upon the oxidation of substrates of the tricarboxylic acid cycle in injured neurons. J Neurochem. 1966 Sep;13(9):849–856. doi: 10.1111/j.1471-4159.1966.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. The response of motor neurones to intramuscular injection of botulinum toxin. J Physiol. 1969 Jun;202(3):611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J. The lateral cervical nucleus in the cat. 3. An electron microscopical study after transection of spinal afferents. Exp Brain Res. 1969;7(1):32–50. doi: 10.1007/BF00236106. [DOI] [PubMed] [Google Scholar]