Abstract
1. Effects of membrane polarization and of reduction in external K and Cl concentration on the inhibitory potential were investigated in the guinea-pig taenia coli.
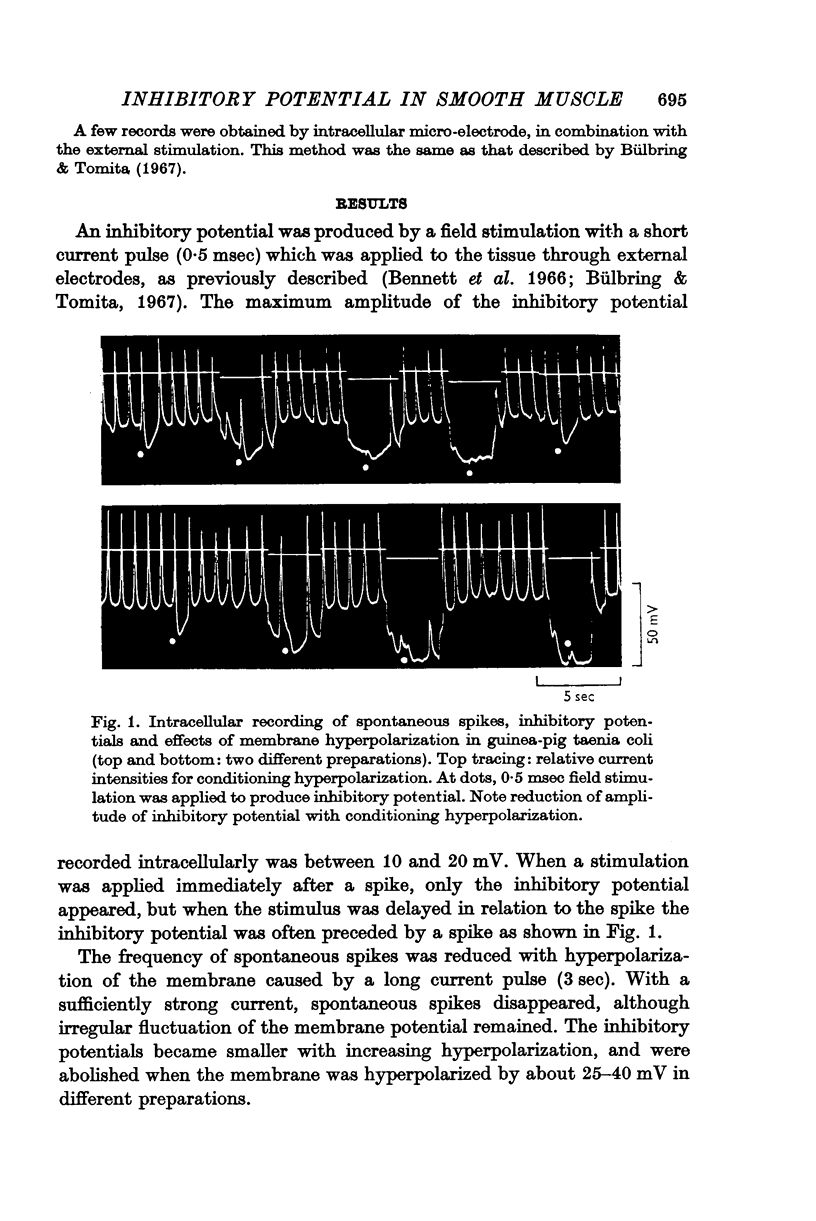
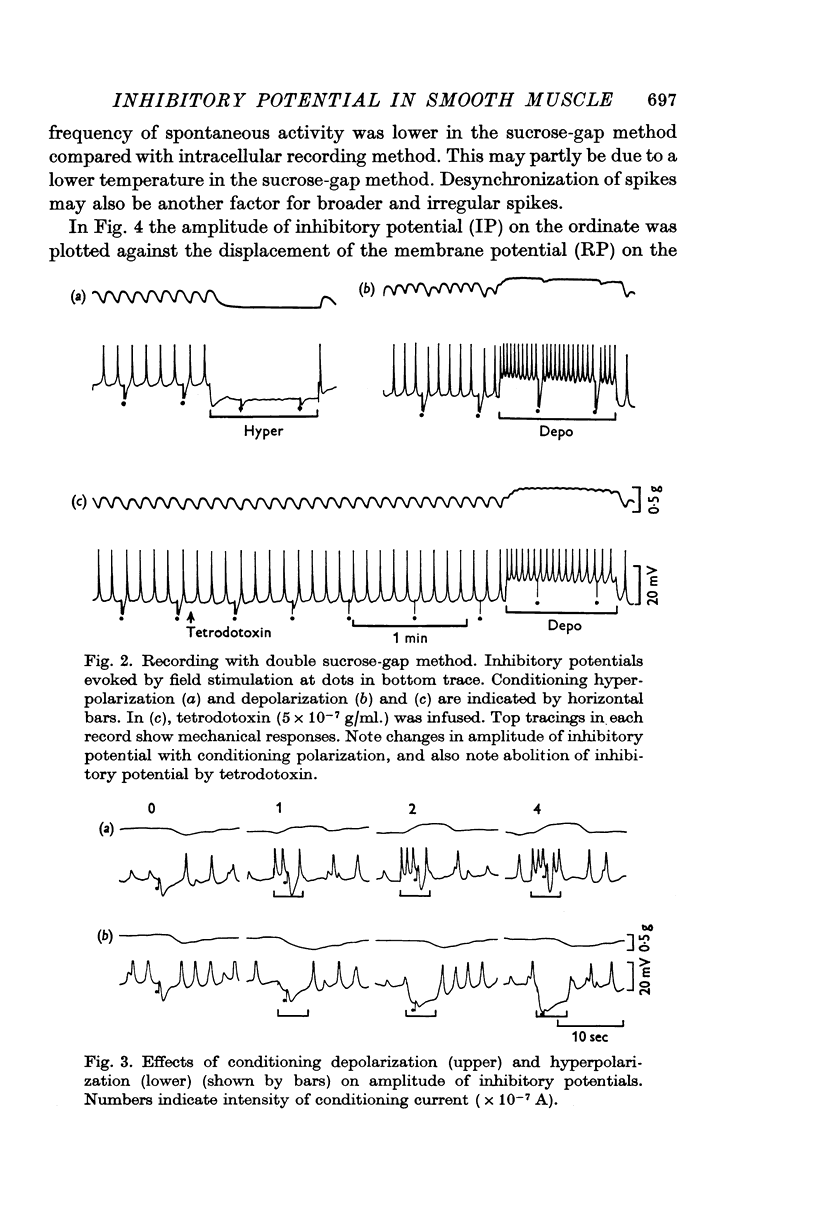
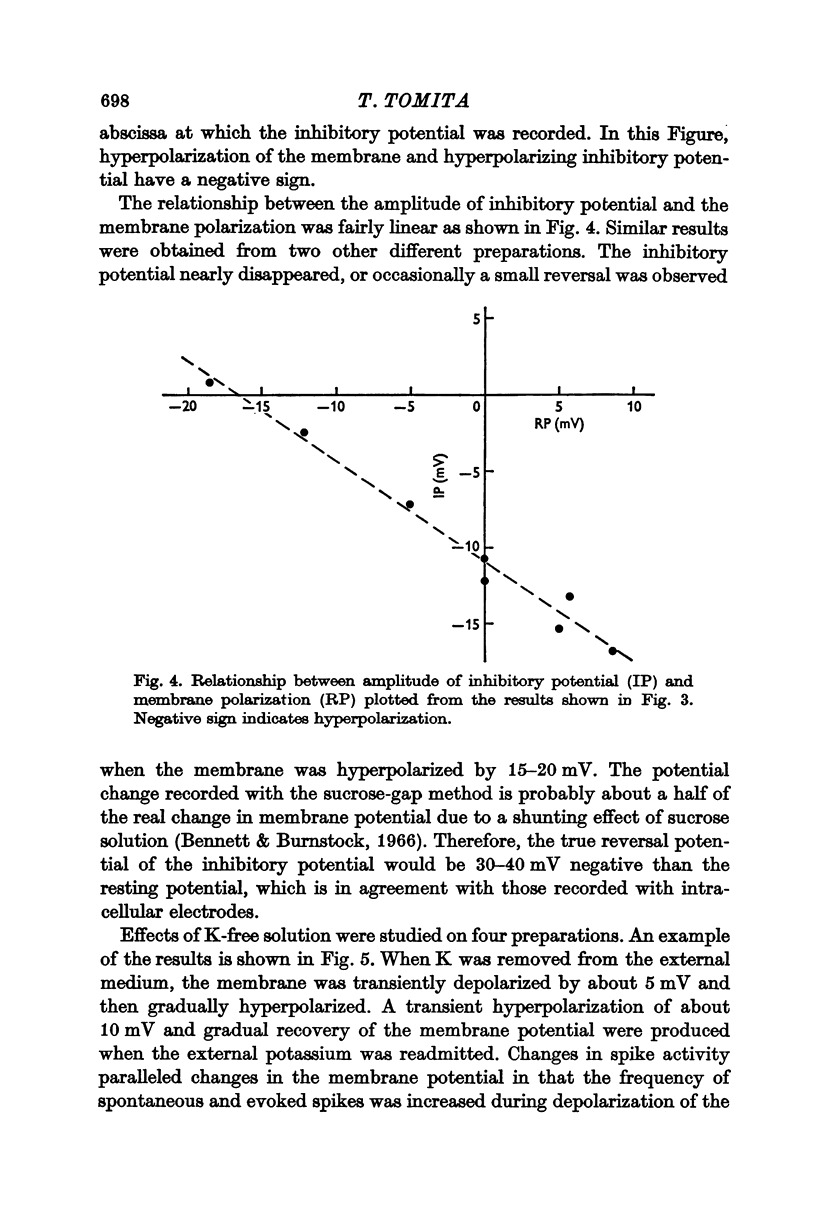
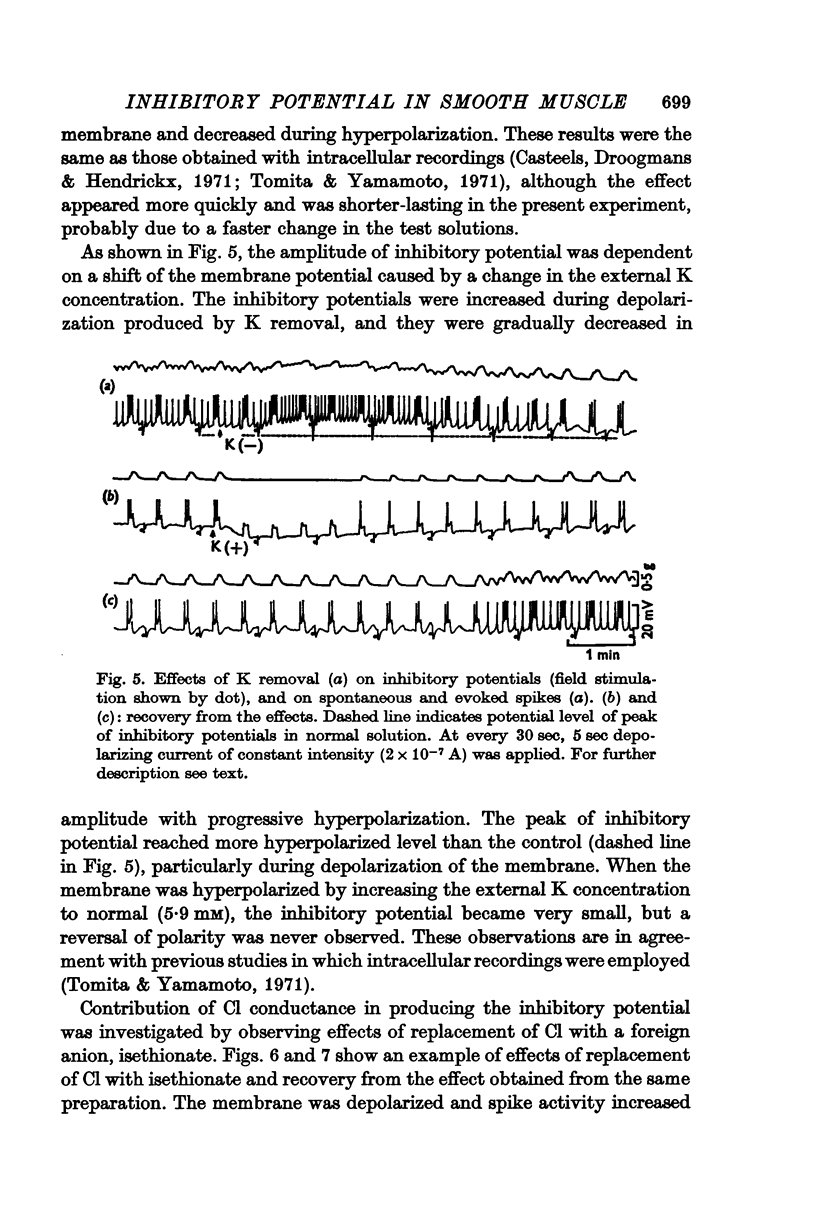
2. Depolarization of the membrane increased the inhibitory potential while hyperpolarization decreased it. The relationship between the degree of membrane polarization and the amplitude of inhibitory potential was linear. The inhibitory potential was abolished or slightly reversed in polarity, when the membrane was hyperpolarized by 25-40 mV in different preparations.
3. Removal of external K ion depolarized the membrane for about 5 min and increased the inhibitory potential more than could be accounted for by the depolarization. Readmission of K transiently hyperpolarized the membrane, probably due to an activation of the Na-K pump, and reduced the inhibitory potential, but no reversal of polarity in the inhibitory potential was observed during this hyperpolarizing phase.
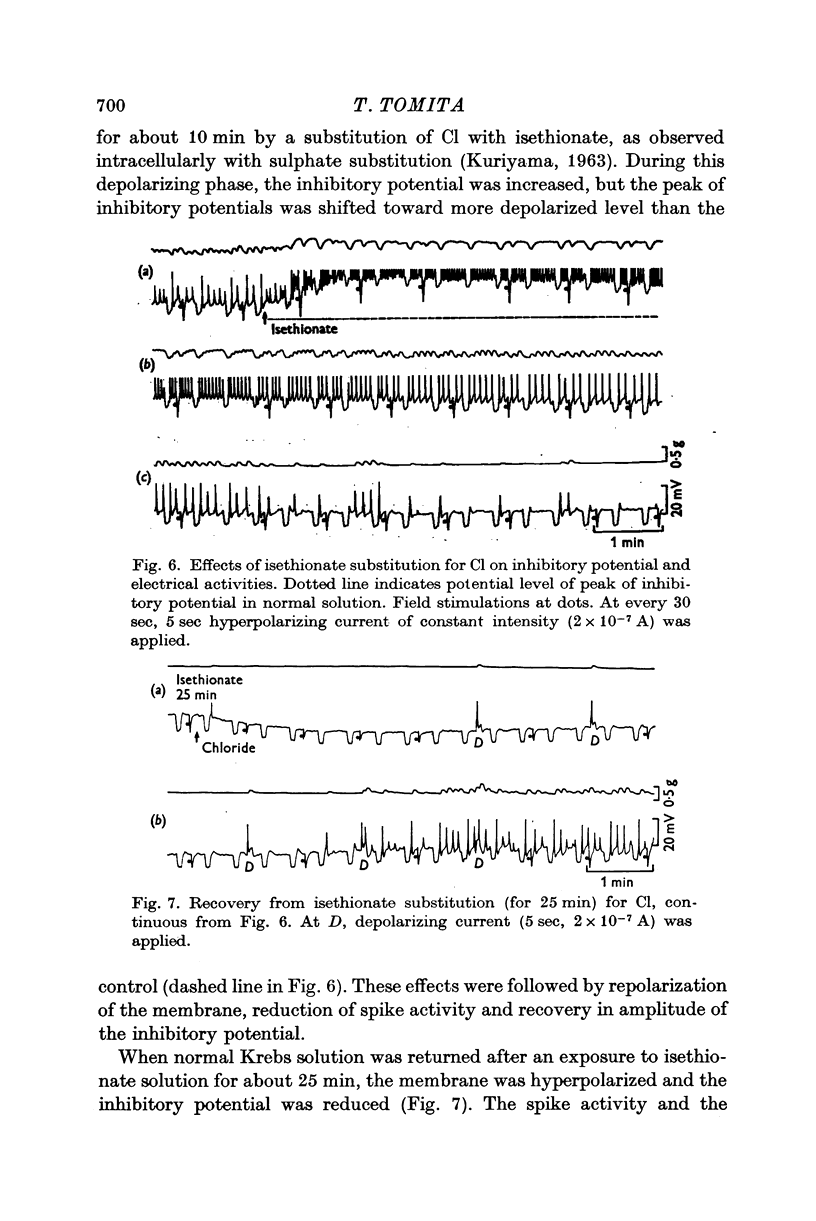
4. The membrane was transiently depolarized when the external Cl concentration was reduced by substituting with isethionate. Hyperpolarization was produced by restoring the external Cl concentration to normal. Changes in the amplitude of inhibitory potentials during alterations in Cl concentration occurred as expected from the shift of the membrane potential.
5. From the results, it is concluded that the membrane conductance is increased during the inhibitory potential, and that an increase in the K permeability is the main factor for hyperpolarization of the membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Crema A. Vagal non-adrenergic inhibition of guinea-pig stomach. J Physiol. 1971 Sep;217(2):259–279. doi: 10.1113/jphysiol.1971.sp009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G. Application of the sucrose-gap method to determine the ionic basis of the membrane potential of smooth muscle. J Physiol. 1966 Apr;183(3):637–648. doi: 10.1113/jphysiol.1966.sp007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Model of the membrane of smooth muscle cells of the guinea-pig taenia coli muscle during transmission from inhibitory and excitatory nerves. Nature. 1966 Sep 10;211(5054):1149–1152. doi: 10.1038/2111149a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Rogers D. C. A study of the innervation of the taenia coli. J Cell Biol. 1967 Jun;33(3):573–596. doi: 10.1083/jcb.33.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. Nerve-mediated excitation and inhibition of the smooth muscle cells of the avian gizzard. J Physiol. 1969 Oct;204(3):669–686. doi: 10.1113/jphysiol.1969.sp008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol. 1967 Apr;189(2):299–315. doi: 10.1113/jphysiol.1967.sp008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE E. P. Resistance values in a syncytium. Aust J Exp Biol Med Sci. 1961 Jun;39:267–274. doi: 10.1038/icb.1961.27. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO S. Membrane changes in crayfish stretch receptor neutron during synaptic inhibition and under action of gamma-aminobutyric acid. J Neurophysiol. 1960 Sep;23:505–515. doi: 10.1152/jn.1960.23.5.505. [DOI] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev. 1966 Jan;46(1):1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]


