Abstract
1. Ciliary activity in the oviduct of the mud puppy Necturus maculosus was monitored by a photometric technique in normal, decalcified, and Triton X-extracted preparations to investigate the regulatory role of calcium ions.
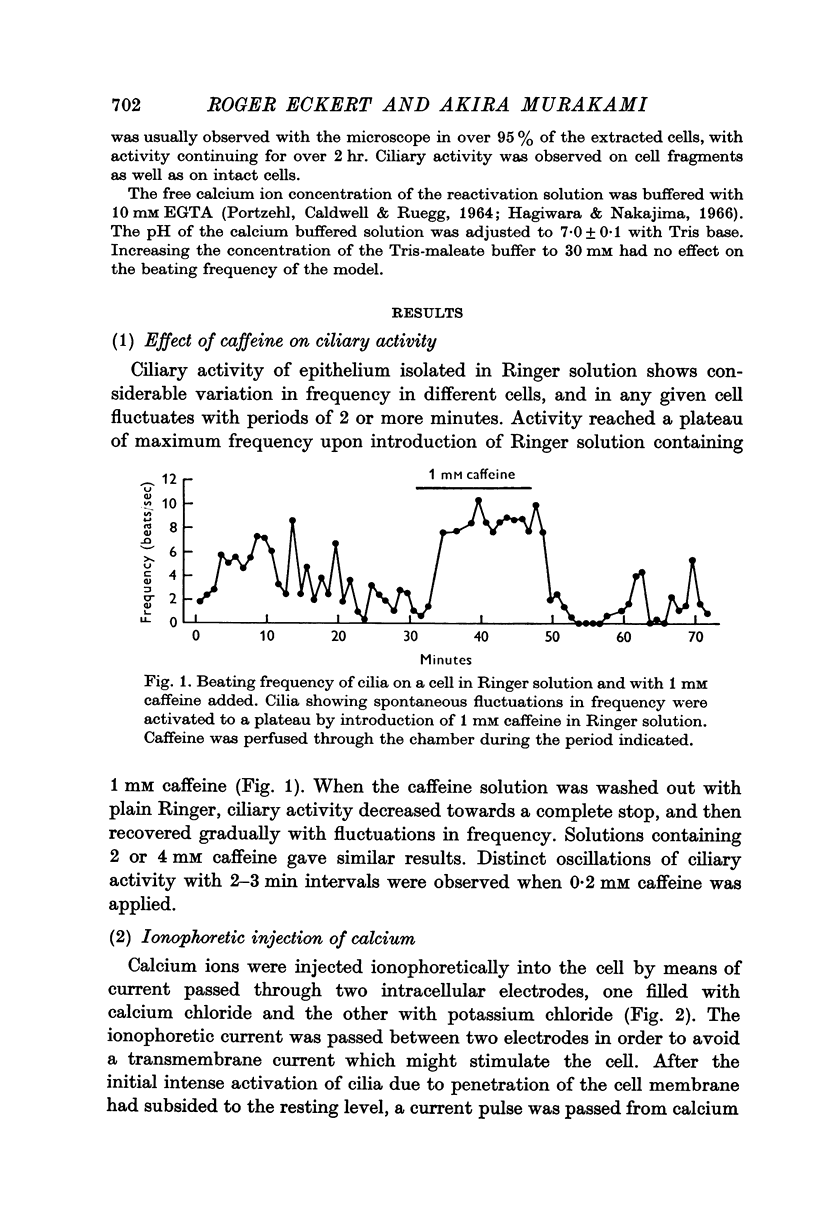
2. The frequency of ciliary beating in the isolated tissue ranged from 0 to 12 beats/sec. The frequency in any one group of cells underwent large cyclical variations with periods of 2 min or more.
3. Frequency of beating reached a maximum and remained at a plateau upon addition of 1 mM caffeine. Beating temporarily ceased upon removal of the caffeine.
4. Ionophoretic injection of calcium ions into an active cell bathed in Ringer solution produced an increased rate of beating. In cells rendered quiescent by prior decalcification with EGTA, injected calcium rapidly restored ciliary activity.
5. Epithelia extracted in Triton X-100 were inactive until reactivated by addition of ATP and magnesium ions. The frequency of beating increased between 0·1 and 4·0 mM ATP in 1 mM magnesium, and between 0·2 and 2·5 mM magnesium in 1 mM ATP.
6. The frequency of beating in the ATP-reactivated, Triton-extracted tissue was independent of the calcium ion concentration.
7. Cells inactivated by decalcification were reactivated by injection of ATP or by extracellular ATP levels as low as 3 × 10-8 M.
8. It is concluded that the frequency of beating depends directly on the concentration of the available energy source, presumably ATP, and that an indirect dependence of beating frequency on calcium concentration in the living tissue results from rate-limiting effects of intracellular calcium on metabolic steps in pathways leading to ATP synthesis.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTHET J., SUTHERLAND E. W., RALL T. W. The assay of glucagon and epinephrine with use of liver homogenates. J Biol Chem. 1957 Nov;229(1):351–361. [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. Studies on the adenosine triphosphatase activity of 14 S and 30 S dynein from cilia of Tetrahymena. J Biol Chem. 1966 Dec 10;241(23):5590–5596. [PubMed] [Google Scholar]
- HOFFMANN-BERLING H. Geisselmodelle und Adenosintriphosphat (ATP). Biochim Biophys Acta. 1955 Jan;16(1):146–154. doi: 10.1016/0006-3002(55)90192-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita H., Murakami A. Control of ciliary motion. Physiol Rev. 1967 Jan;47(1):53–82. doi: 10.1152/physrev.1967.47.1.53. [DOI] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. On the control of glycogenolysis in mammalian nervous tissue by calcium. J Physiol. 1971 Jan;212(2):503–517. doi: 10.1113/jphysiol.1971.sp009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE K. E., MILTON A. S., GOSSELIN R. E. Effect of 5-hydroxytryptamine on the respiration of excised lamellibranch gill. Br J Pharmacol Chemother. 1961 Oct;17:278–285. doi: 10.1111/j.1476-5381.1961.tb01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Hasker J. R. Effect of caffeine on calcium in subcellular fractions of cardiac muscle. Am J Physiol. 1966 Oct;211(4):950–954. doi: 10.1152/ajplegacy.1966.211.4.950. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- VORHAUS E. F., DEYRUP I. J. The effect of adenosinetriphosphate on the cilia of the pharyngeal mucosa of the frog. Science. 1953 Nov 6;118(3071):553–554. doi: 10.1126/science.118.3071.553. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


