Abstract
1. The action of peripheral nerve volleys on the polarization of presynaptic terminals of inactive sensory fibres in cat lumbar spinal cord has been investigated by recording (a) the dorsal root potential (DRP), (b) intracellular changes in polarization of single preterminal axons (PAD or PAH), and (c) changes in excitability of populations of preterminal axons.
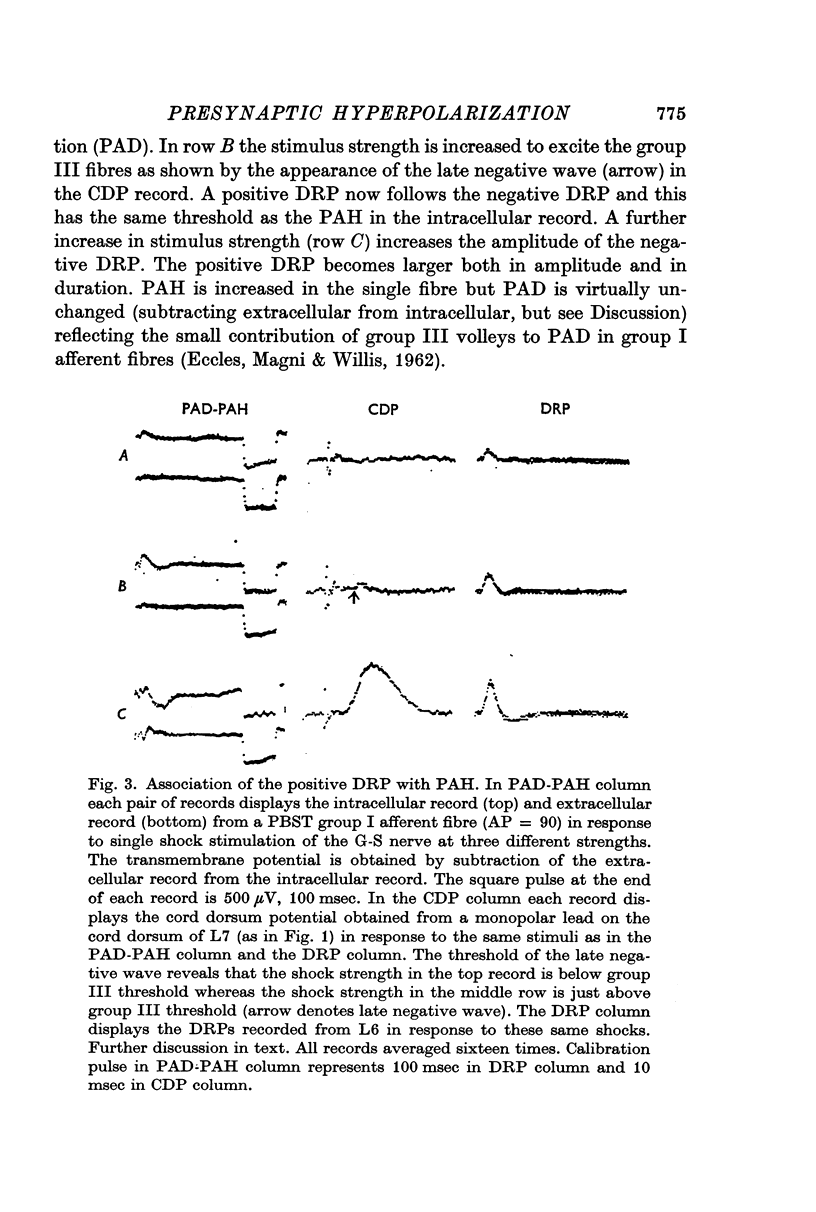
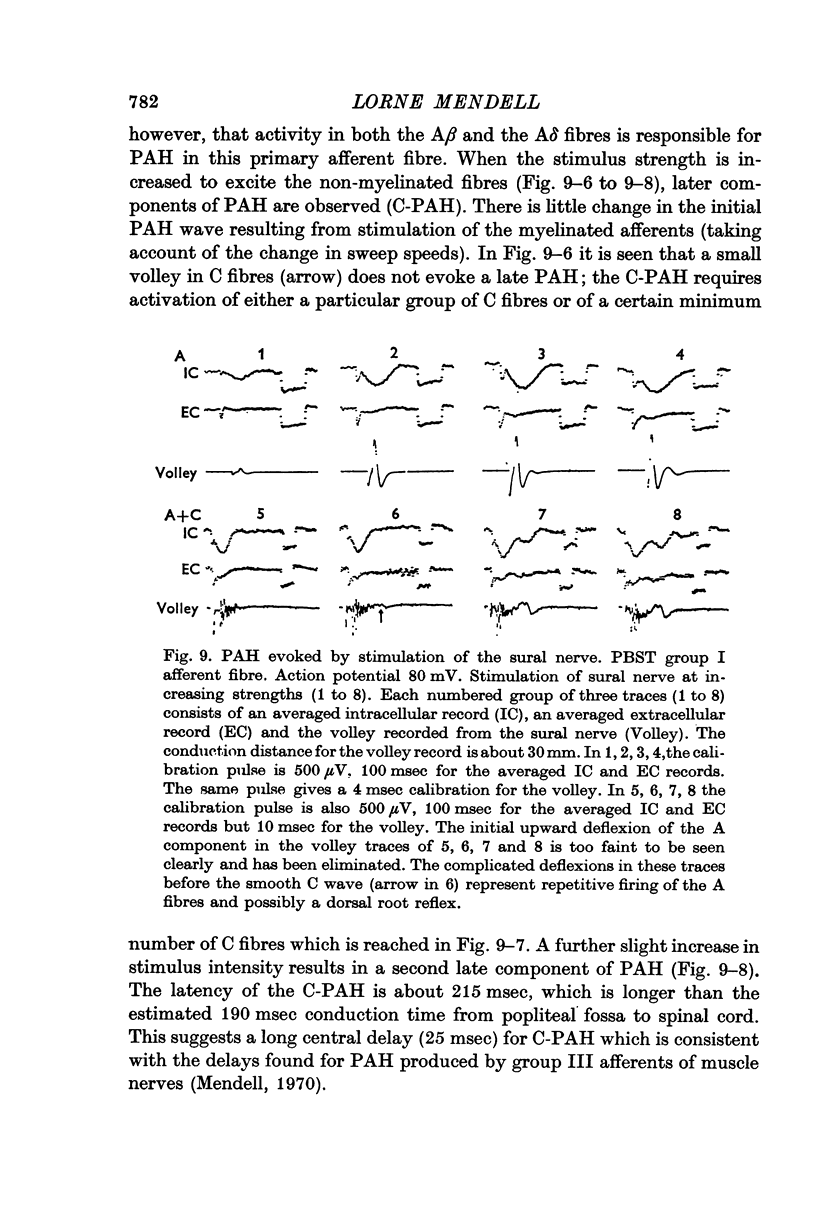
2. Presynaptic hyperpolarization (positive DRP-PAH) can be evoked by stimulation of muscle group III afferents as well as by volleys in cutaneous Aβ, Aδ and C afferents. These volleys can also produce presynaptic depolarization (negative DRP-PAD).
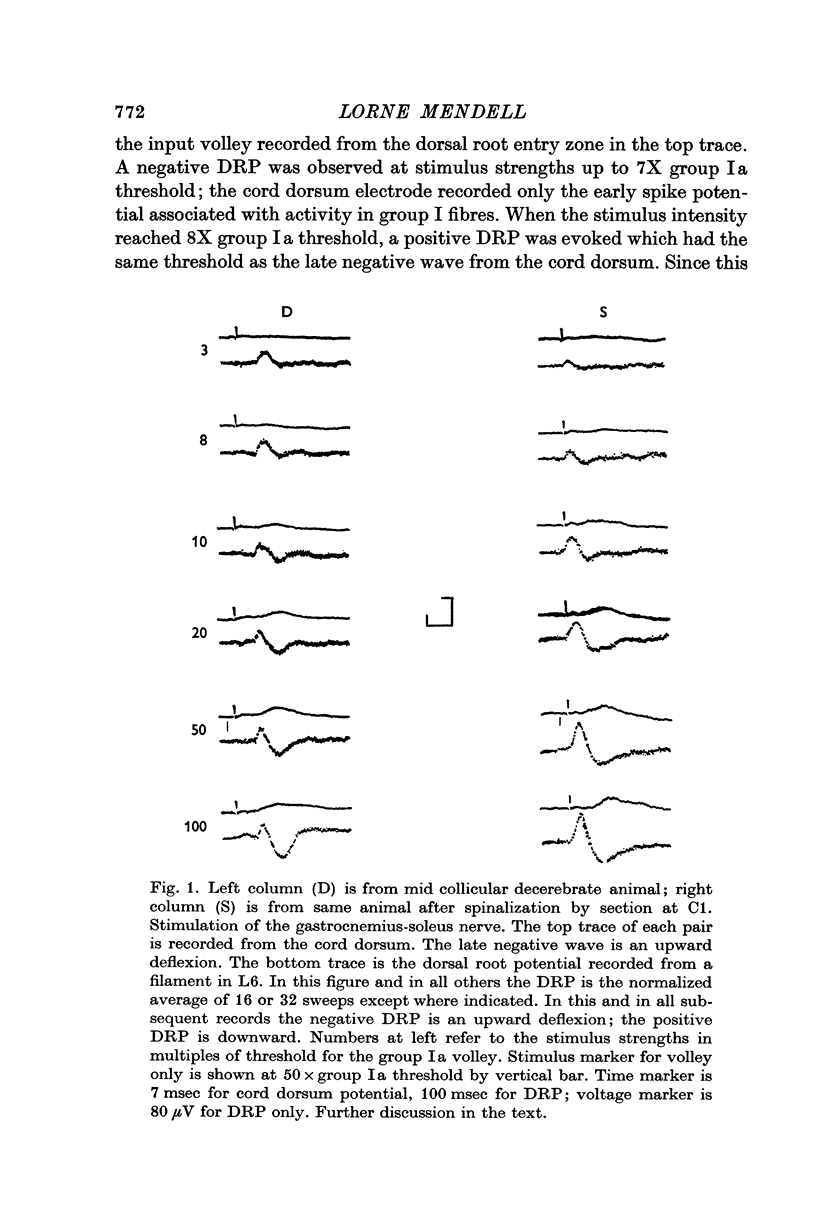
3. The positive DRP is observed in the decerebrate state and increases in amplitude following spinalization.
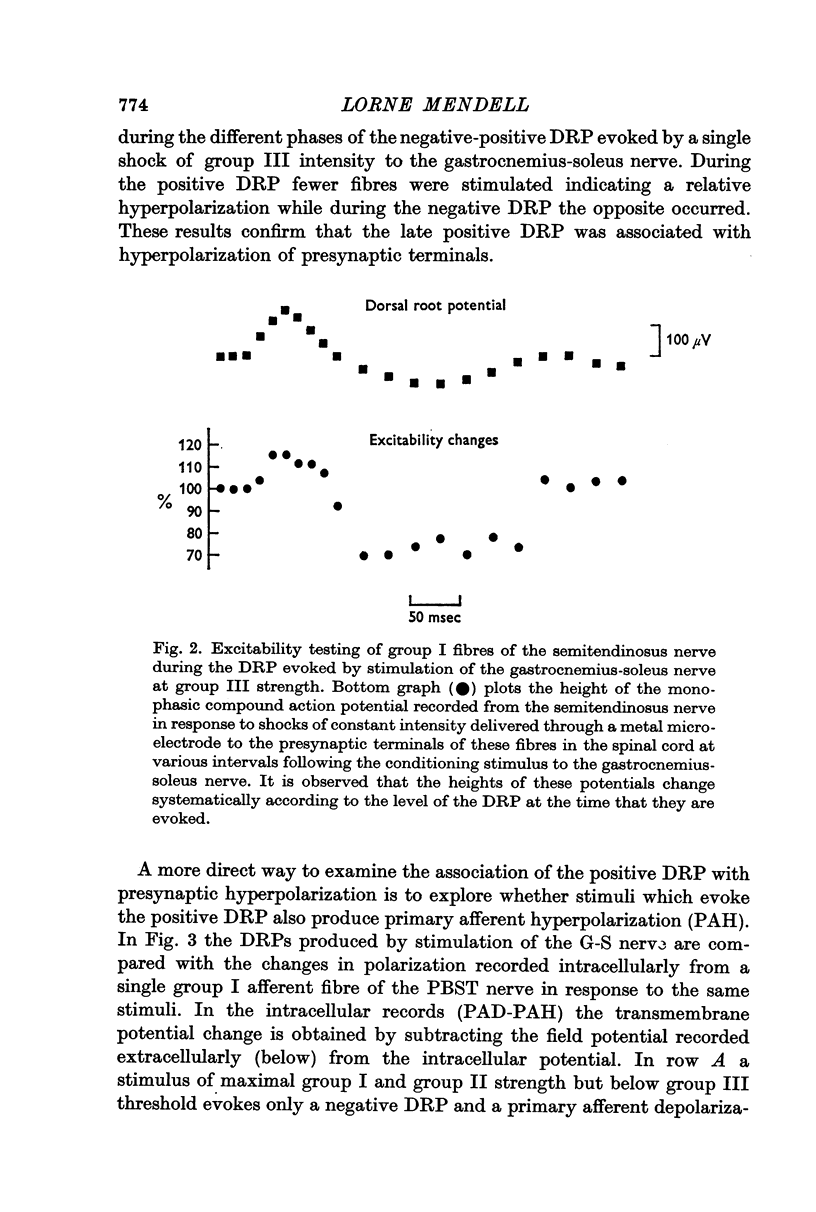
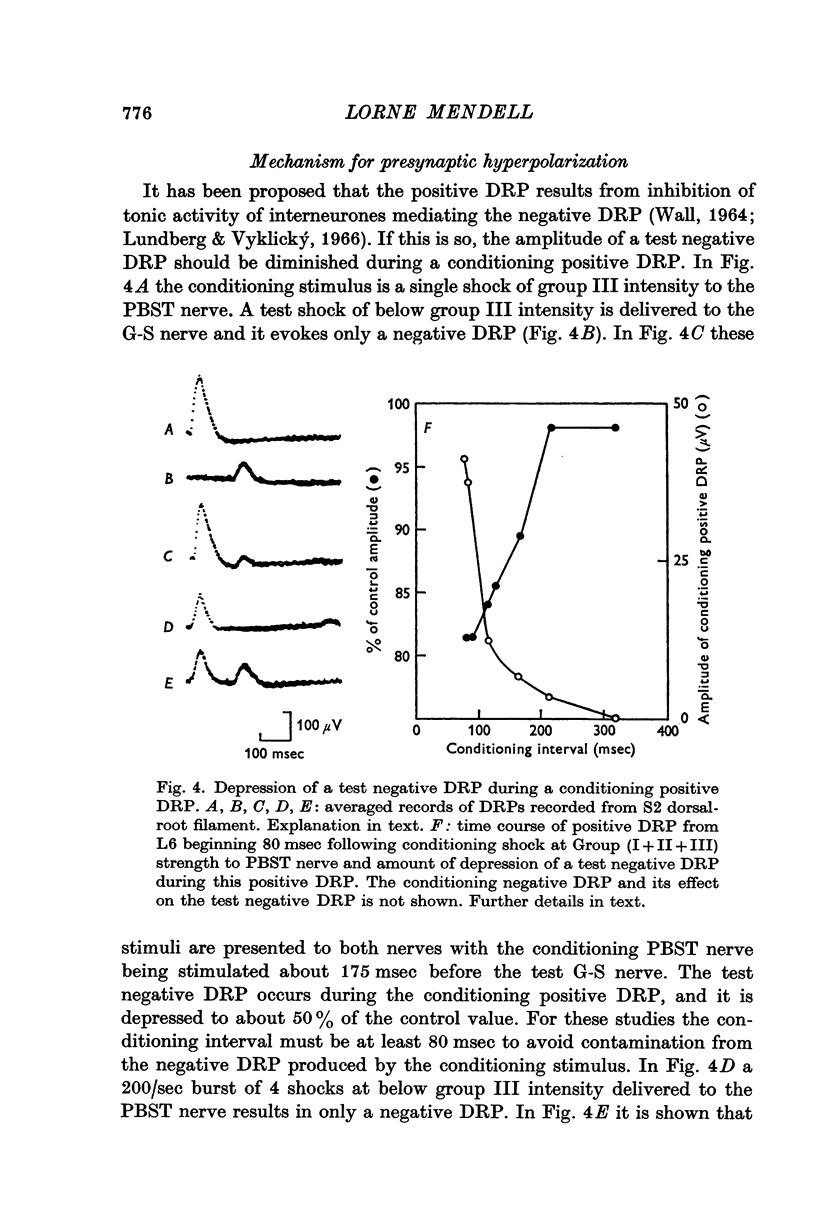
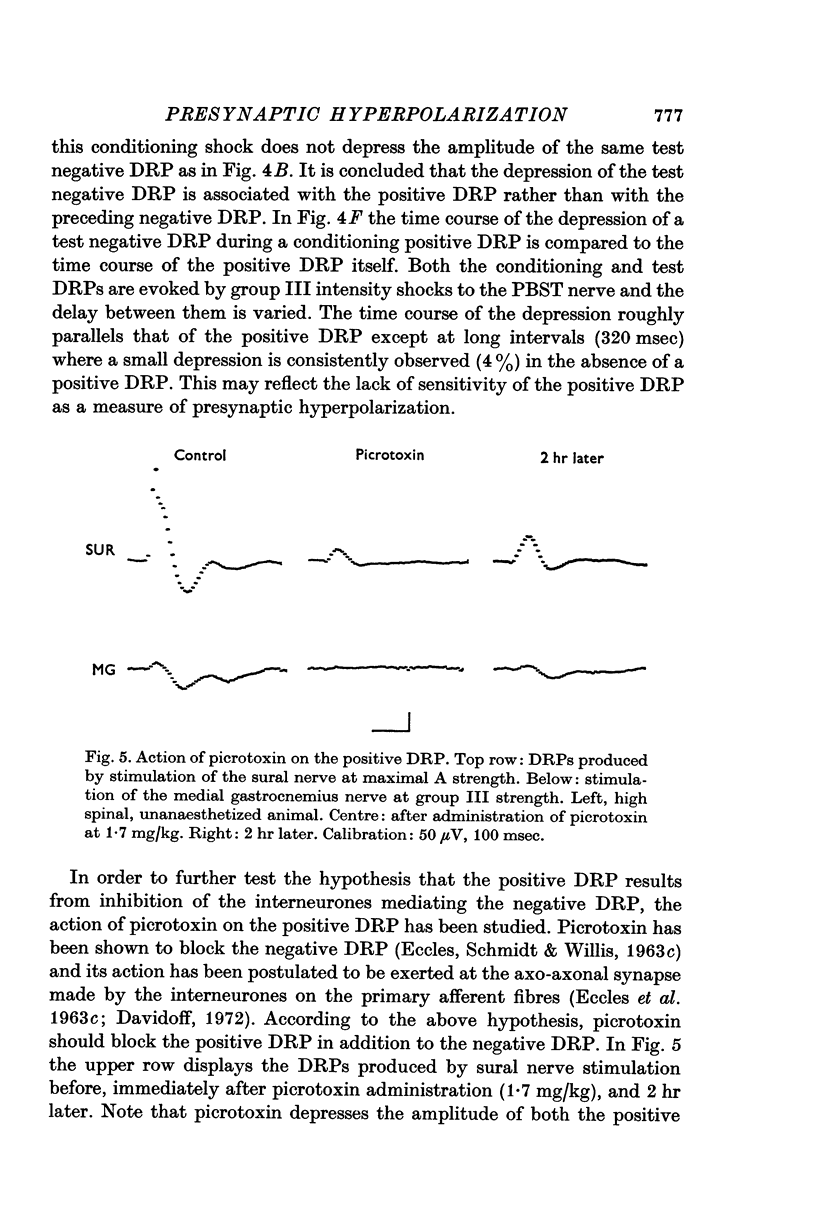
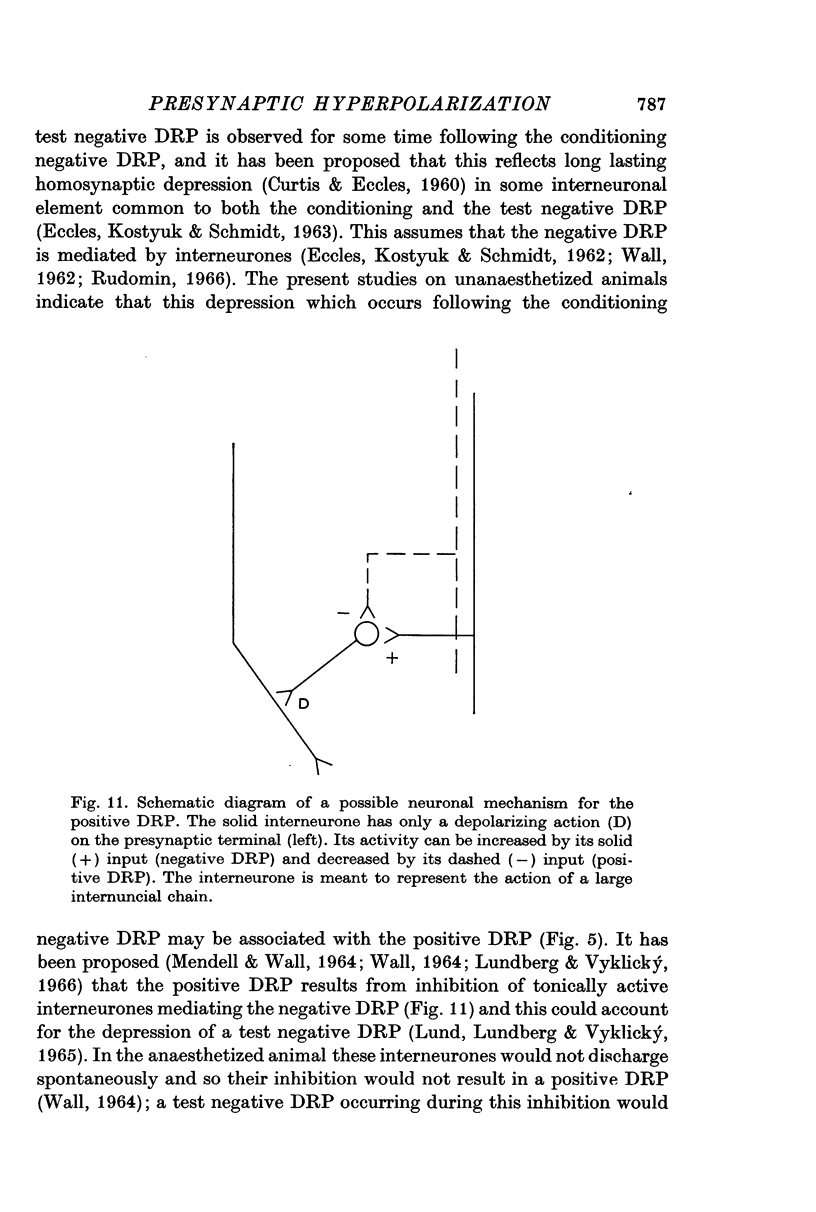
4. Picrotoxin blocks the positive DRP at the same dosages required to block the negative DRP. Test negative DRPs are depressed during a conditioning positive DRP. These results are used to support earlier suggestions that the positive DRP results from inhibition of interneurones mediating the negative DRP.
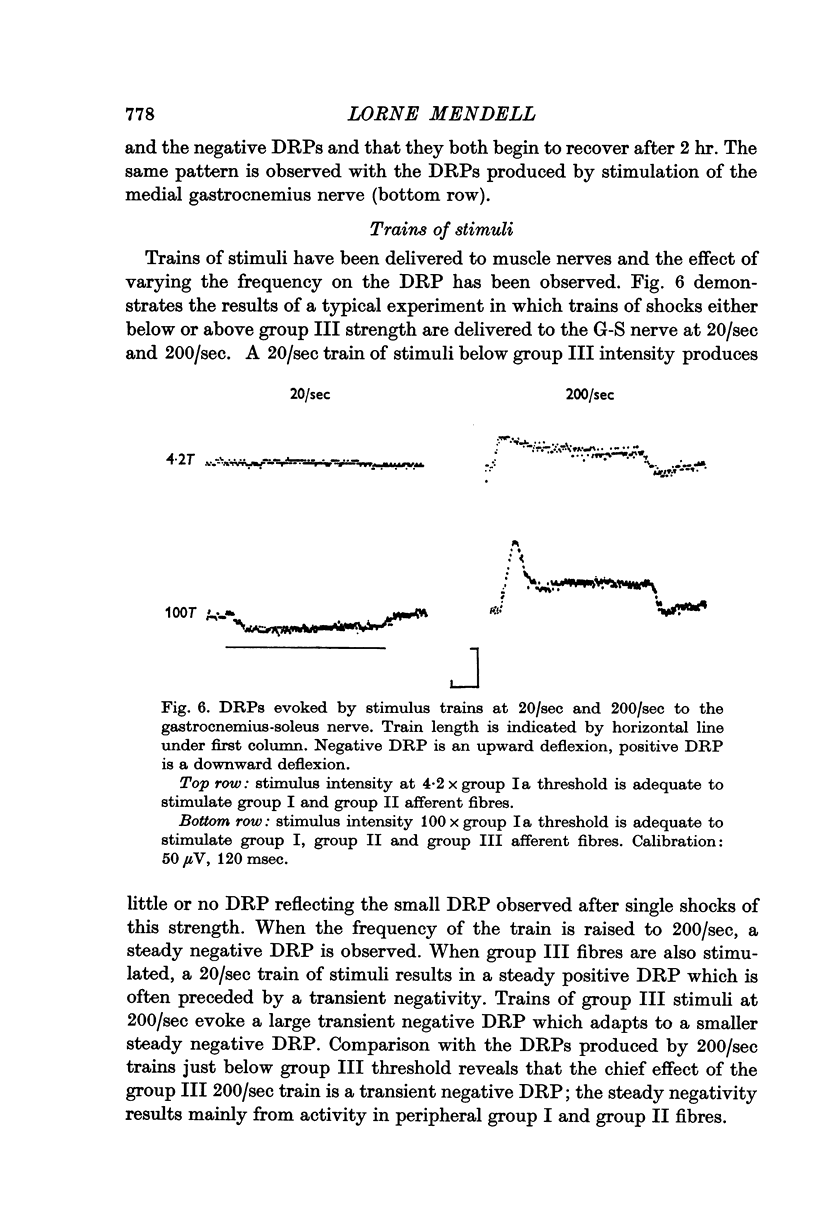
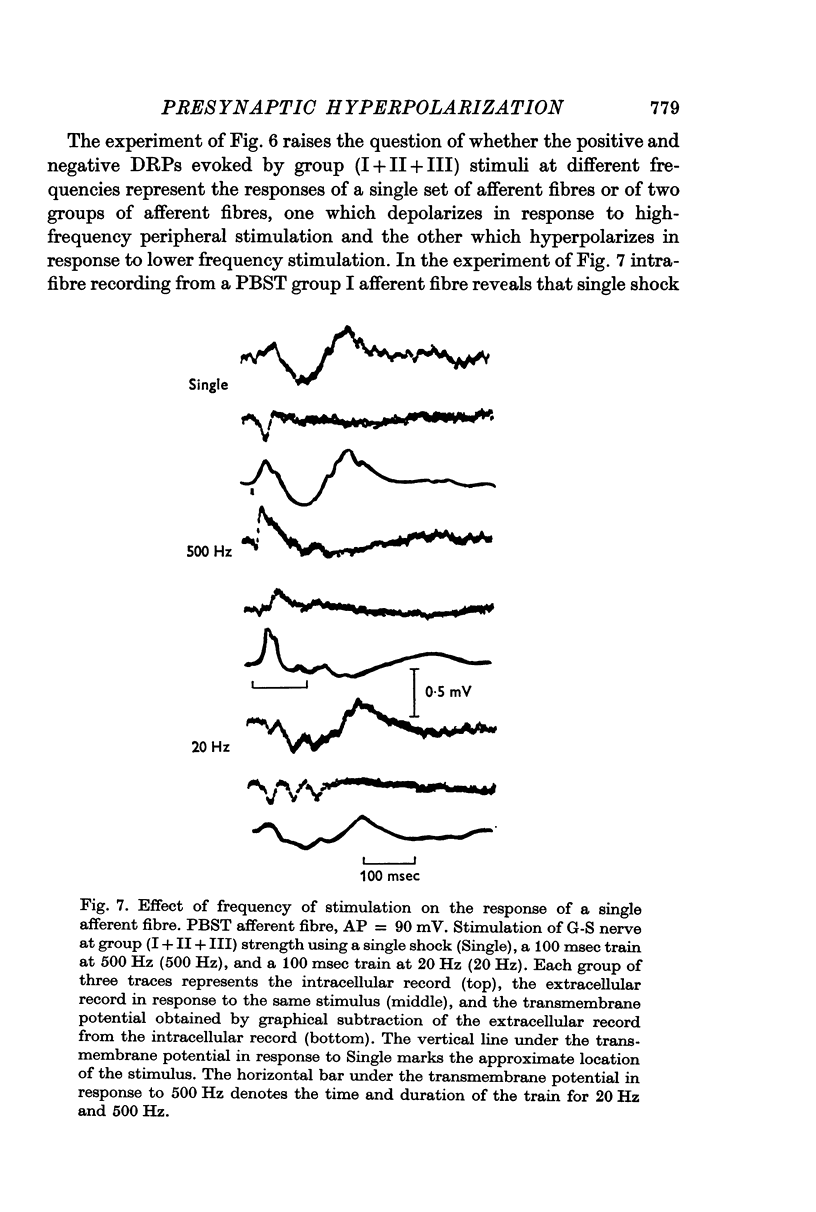
5. Trains of group III stimuli at 20/sec evoke a steady positive DRP. Trains of the same intensity at 200/sec evoke a phasic negative DRP. This frequency dependence is observed for PAD and PAH in single sensory axons.
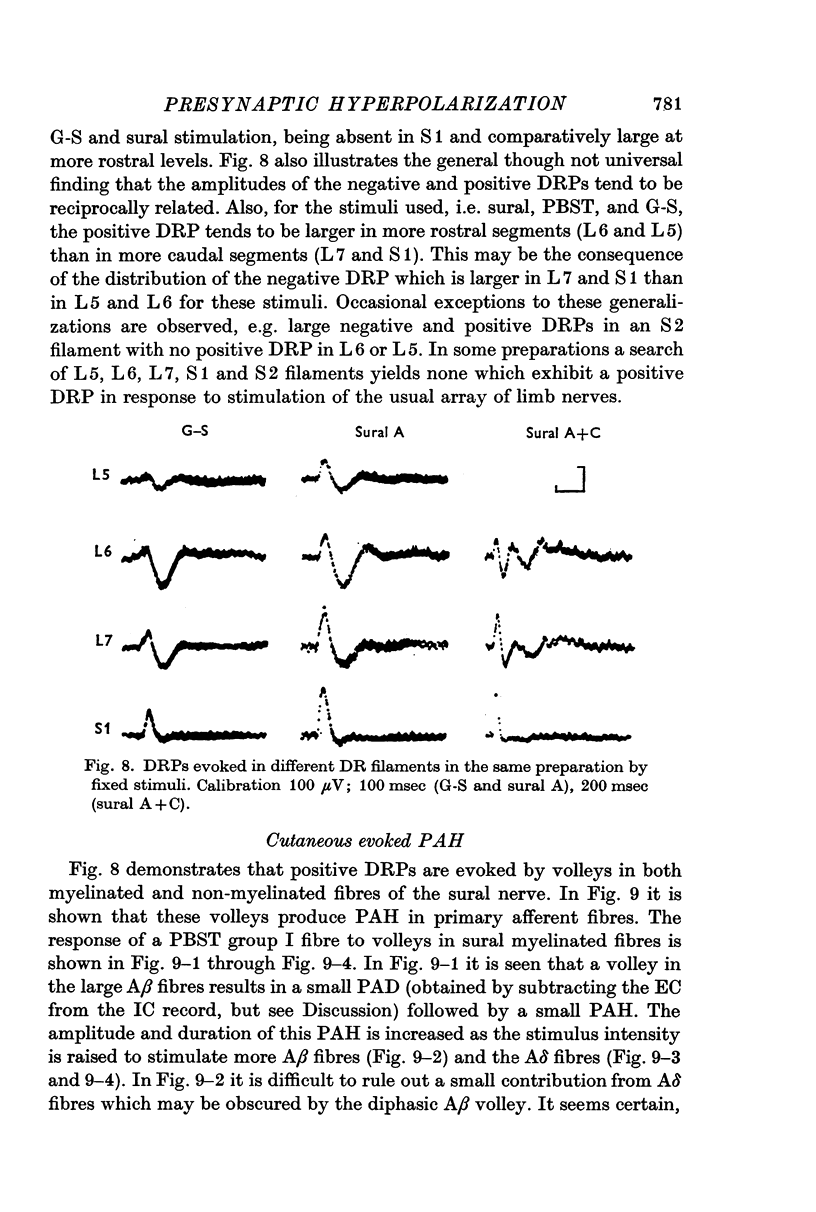
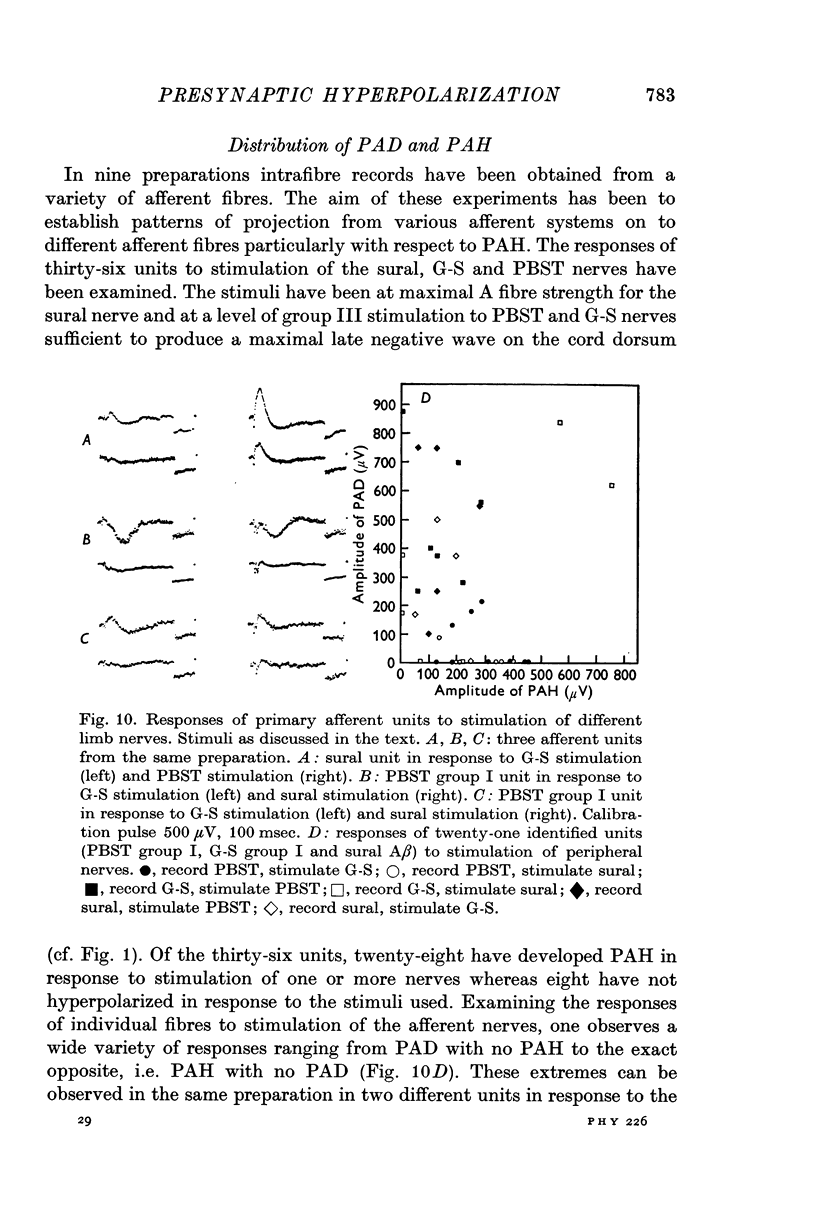
6. The DRPs recorded from different dorsal root filaments in response to a given stimulus vary widely in the ratio of negative to positive DRP.
7. Intracellular recording from single axons reveals that the same stimuli evoke widely varying ratios of PAD and PAH.
8. Stimulation of FRA evokes PAH > PAD in PBST group I afferents, PAD > PAH in sural A fibres and intermediate effects in G-S group I units.
9. It is suggested that activation of flexor reflex afferents may selectively potentiate the synaptic efficacy of large muscle afferents mediating the flexor reflex rather than large skin afferents or large afferents from extensor muscles.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rudomin P., Vyklický L., Zajac F. E., 3rd Primary afferent depolarization and flexion reflexes produced by radiant heat stimulation of the skin. J Physiol. 1971 Feb;213(1):185–214. doi: 10.1113/jphysiol.1971.sp009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER D., ENGBERG I., FUNKENSTEIN H., LUNDBERG A. DECEREBRATE CONTROL OF REFLEXES TO PRIMARY AFFERENTS. Acta Physiol Scand. 1963 Dec;59:424–437. doi: 10.1111/j.1748-1716.1963.tb02758.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Cook W. A., Jr, Pompeiano O. Cerebellar inhibitory control of the vestibular reflex pathways to primary afferents. Arch Ital Biol. 1969 Aug;107(3):341–364. [PubMed] [Google Scholar]
- Chan S. H., Barnes C. D. Presynaptic facilitation: positive dorsal root potentials evoked from brain stem reticular formation in lumbar cord. Brain Res. 1971 Apr 16;28(1):176–179. doi: 10.1016/0006-8993(71)90535-x. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Central pathways responsible for depolarization of primary afferent fibres. J Physiol. 1962 May;161:237–257. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Presynaptic inhibition of the central actions of flexor reflex afferents. J Physiol. 1962 May;161:258–281. doi: 10.1113/jphysiol.1962.sp006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K. Presynaptic changes associated with post-tetanic potentiation in the spinal cord. J Physiol. 1959 Dec;149:274–287. doi: 10.1113/jphysiol.1959.sp006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R. F., WILLIS W. D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol. 1962 May;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Dorsal root potentials and ventral root reflexes evoked by nonmyelinated fibers. Science. 1968 Dec 6;162(3858):1140–1142. doi: 10.1126/science.162.3858.1140. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGBARTH K. E., NAESS K. Reflex effects of tetanic stimulation of different afferent fibre-systems in the hind limb of the cat. Acta Physiol Scand. 1950 Dec;21(4):336–361. doi: 10.1111/j.1748-1716.1950.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Hodge C. J., Jr Potential changes inside central afferent terminals secondary to stimulation of large- and small-diameter peripheral nerve fibers. J Neurophysiol. 1972 Jan;35(1):30–43. doi: 10.1152/jn.1972.35.1.30. [DOI] [PubMed] [Google Scholar]
- Jänig W., Zimmermann M. Presynaptic depolarization of myelinated afferent fibres evoked by stimulation of cutaneous C fibres. J Physiol. 1971 Apr;214(1):29–50. doi: 10.1113/jphysiol.1971.sp009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Intracellular potential changes of primary afferent nerve fibers in spinal cords of cats. J Neurophysiol. 1956 Sep;19(5):375–392. doi: 10.1152/jn.1956.19.5.375. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C. Electrotonus in dorsal nerve roots. Cold Spring Harb Symp Quant Biol. 1952;17:203–219. doi: 10.1101/sqb.1952.017.01.020. [DOI] [PubMed] [Google Scholar]
- Lund S., Lundberg A., Vyklický L. Inhibitory action from the flexor reflex afferents on transmission to Ia afferents. Acta Physiol Scand. 1965 Aug;64(4):345–355. doi: 10.1111/j.1748-1716.1965.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Vyklický L. Inhibition of transmission to primary afferents by electrical stimulation of the brain stem. Arch Ital Biol. 1966 Mar;104(1):86–97. [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. PRESYNAPTIC HYPERPOLARIZATION: A ROLE FOR FINE AFFERENT FIBRES. J Physiol. 1964 Aug;172:274–294. doi: 10.1113/jphysiol.1964.sp007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mendell L. Positive dorsal root potentials produced by stimulaton of small diameter muscle afferents. Brain Res. 1970 Mar 3;18(2):375–379. doi: 10.1016/0006-8993(70)90339-2. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Pharmacological evidence for the existence of interneurons mediating primary afferent depolarization in the solitary tract nucleus of the cat. Brain Res. 1966 Aug;2(2):181–183. doi: 10.1016/0006-8993(66)90023-0. [DOI] [PubMed] [Google Scholar]
- Scibetta C. J., King R. B. Hyperpolarizing influence of trigeminal nucleus caudalis on primary afferent preterminals in trigeminal nucleus oralis. J Neurophysiol. 1969 Mar;32(2):229–238. doi: 10.1152/jn.1969.32.2.229. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Dubner R. Presynaptic hyperpolarization of fibers projecting to trigeminal brain stem and thalamic nuclei. Brain Res. 1970 Aug 12;22(1):121–125. doi: 10.1016/0006-8993(70)90407-5. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Evoked sustained focal potentials and membrane potential of neurons and of unresponsive cells of the spinal cord. J Neurophysiol. 1970 Jul;33(4):562–582. doi: 10.1152/jn.1970.33.4.562. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL P. D. The origin of a spinal-cord slow potential. J Physiol. 1962 Dec;164:508–526. doi: 10.1113/jphysiol.1962.sp007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. F., King R. B. Excitability changes in trigeminal primary afferent fibers in response to noxious and nonnoxious stimuli. J Neurophysiol. 1972 Jan;35(1):87–95. doi: 10.1152/jn.1972.35.1.87. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Dorsal root potentials after C-fiber stimulation. Science. 1968 May 24;160(3830):896–898. doi: 10.1126/science.160.3830.896. [DOI] [PubMed] [Google Scholar]


