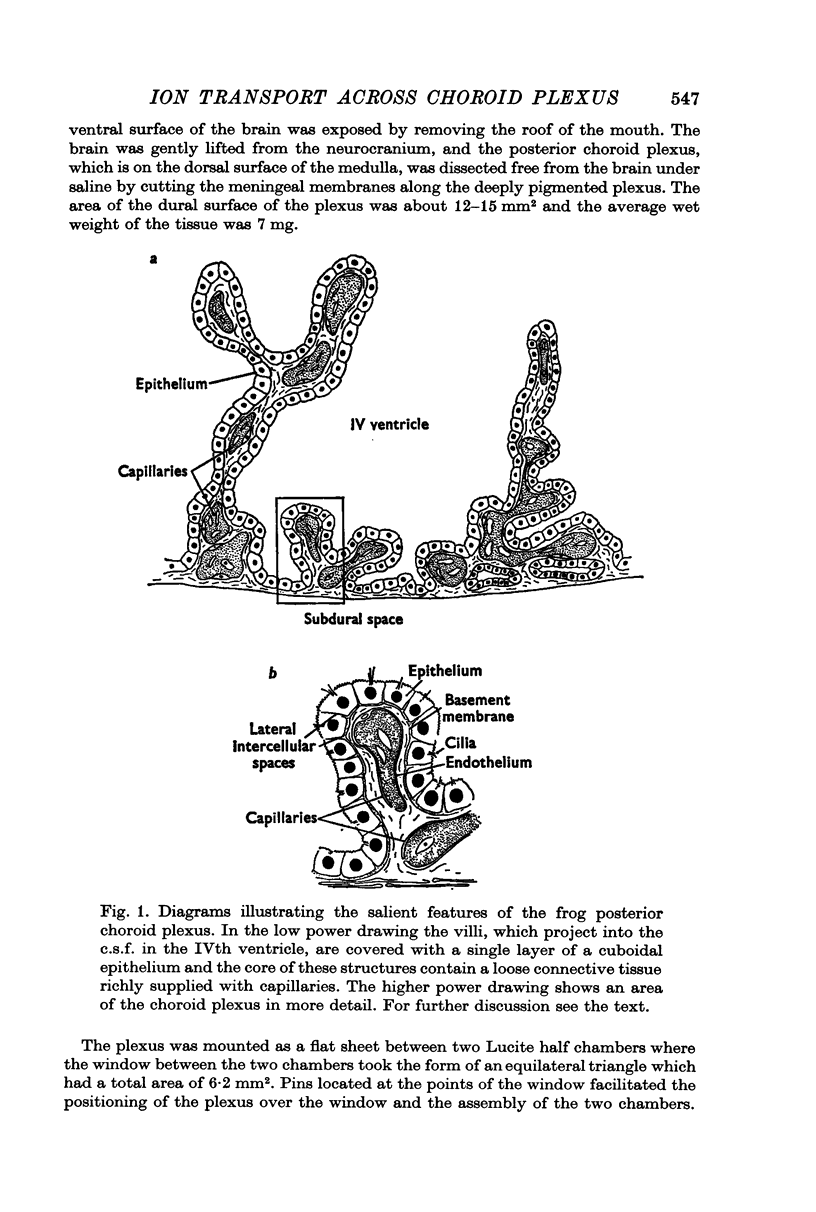
Abstract
1. Mechanisms of ion transport across the choroidal epithelium were investigated using an in vitro preparation of the frog choroid plexus.
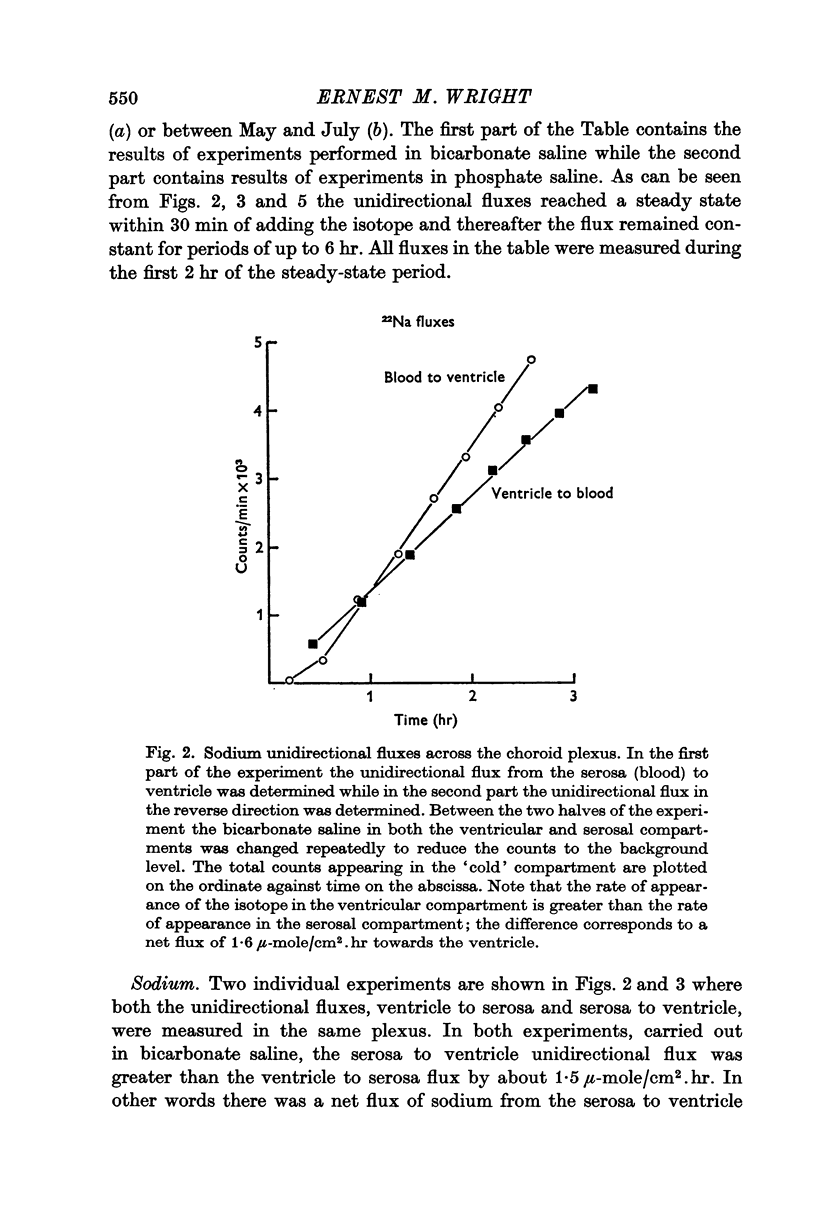
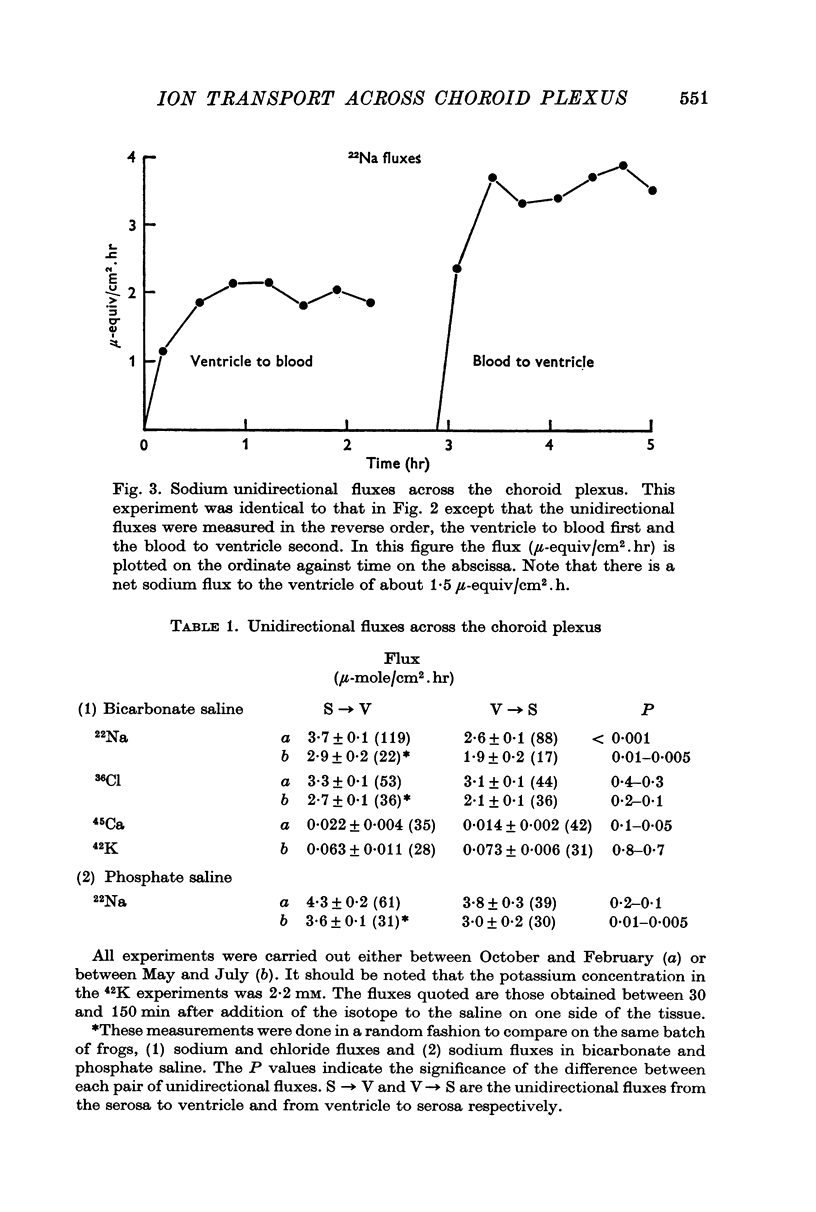
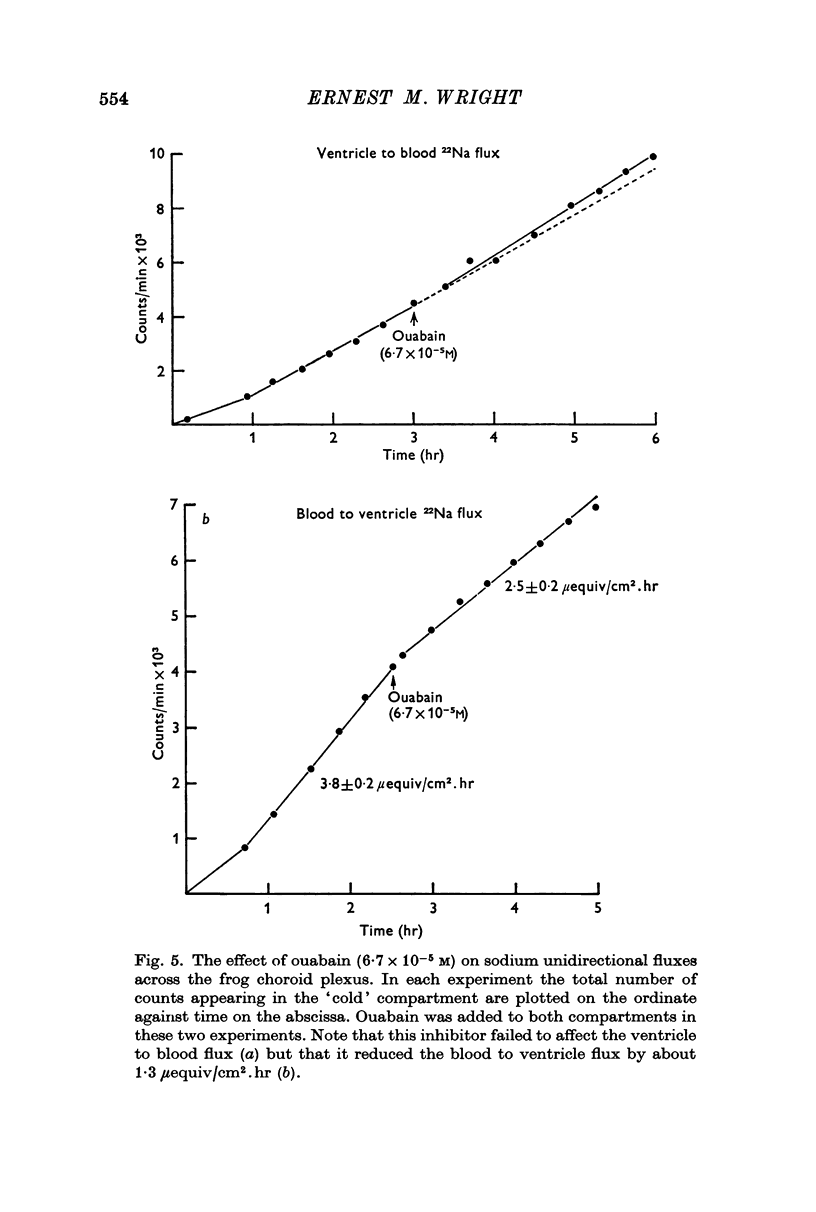
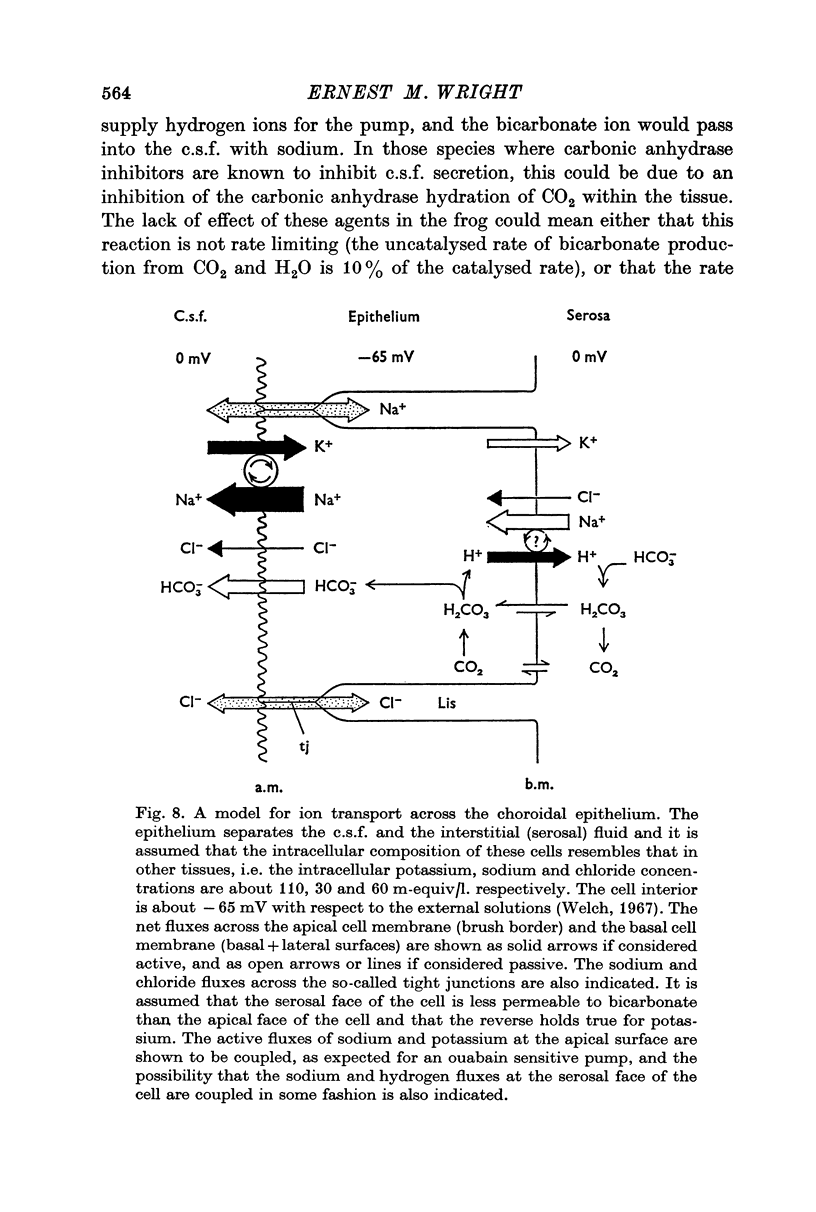
2. Sodium was actively transported across the plexus from the vascular to the ventricular surface by an ouabain sensitive electrically silent pump. As in other epithelial membranes the rate of sodium transport was stimulated by the presence of bicarbonate ions in the Ringer solutions. Chloride and bicarbonate ions accompany the net flux of sodium across this tissue.
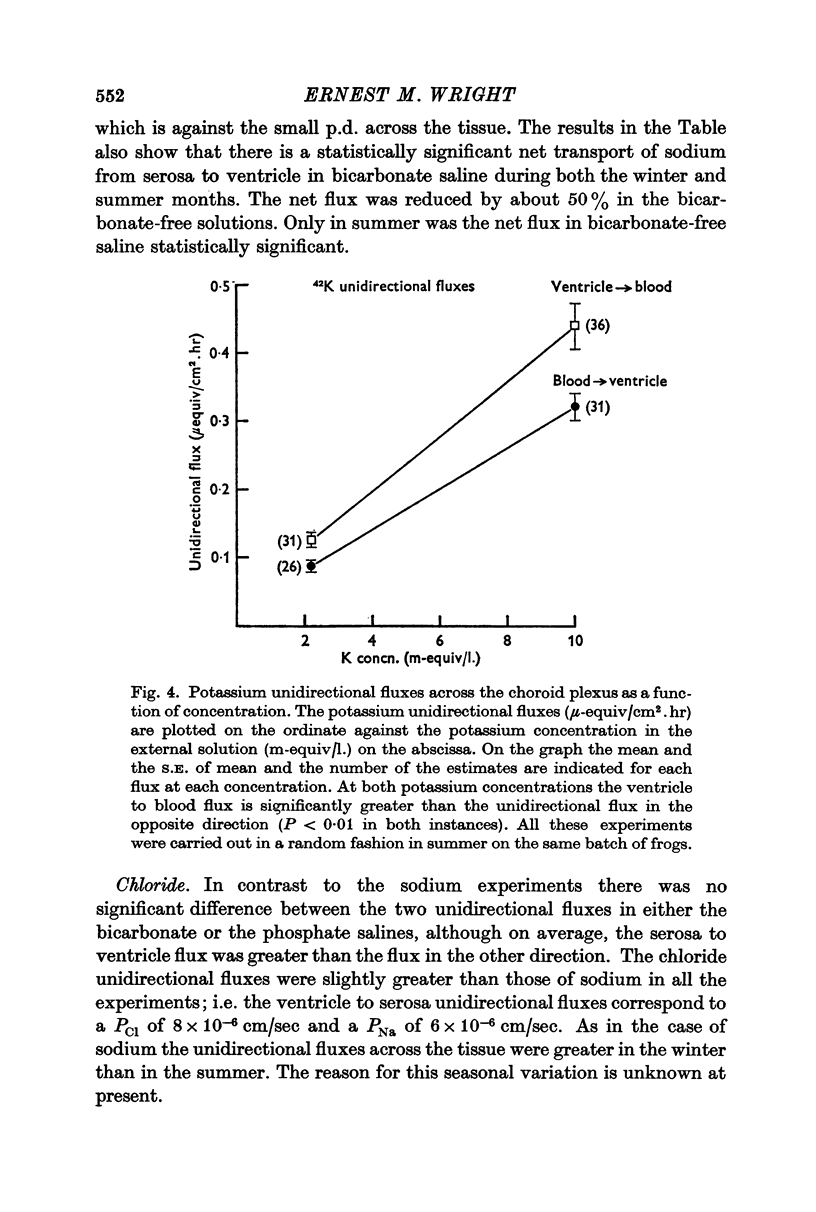
3. Some experiments suggest that potassium is actively transported from the ventricular to the serosal surface, and that the rate of transport is a function of the extracellular potassium concentration.
4. No evidence was obtained to suggest that calcium is actively transported across this tissue in either direction.
5. Diamox, ethoxyzolamide, pitocin, pitressin, hydrocortisone, amiloride, spironolactone and anoxia all failed to influence sodium transport.
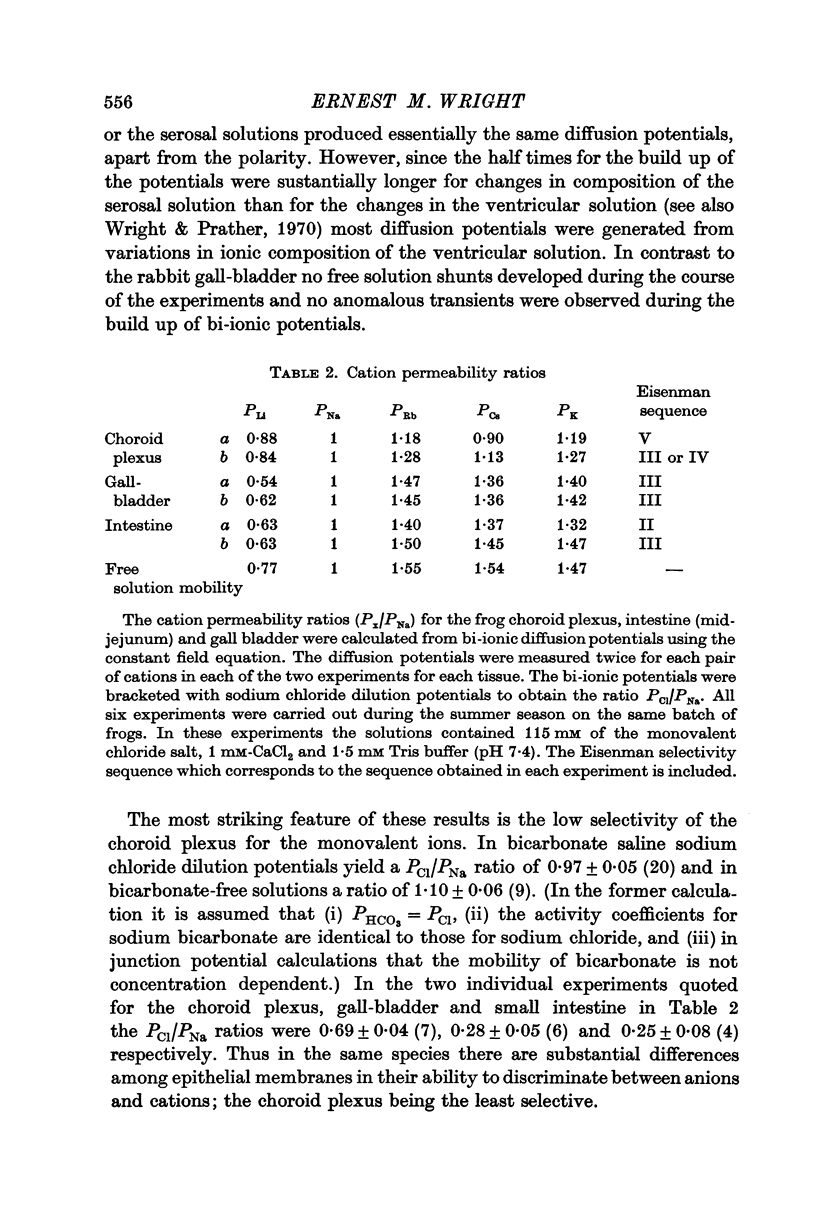
6. The sequence of passive ion permeation across the plexus was PRb ∼ PK > PCs ∼ PNa ∼ PCl ∼ PHCO3 > PLi as deduced from diffusion potential measurements. At least for Na, K and Cl there was a good correlation between the permeability coefficients derived from unidirectional flux measurements and from electrical parameters. This indicates that exchange diffusion is unimportant as a mechanism for passive ion transport.
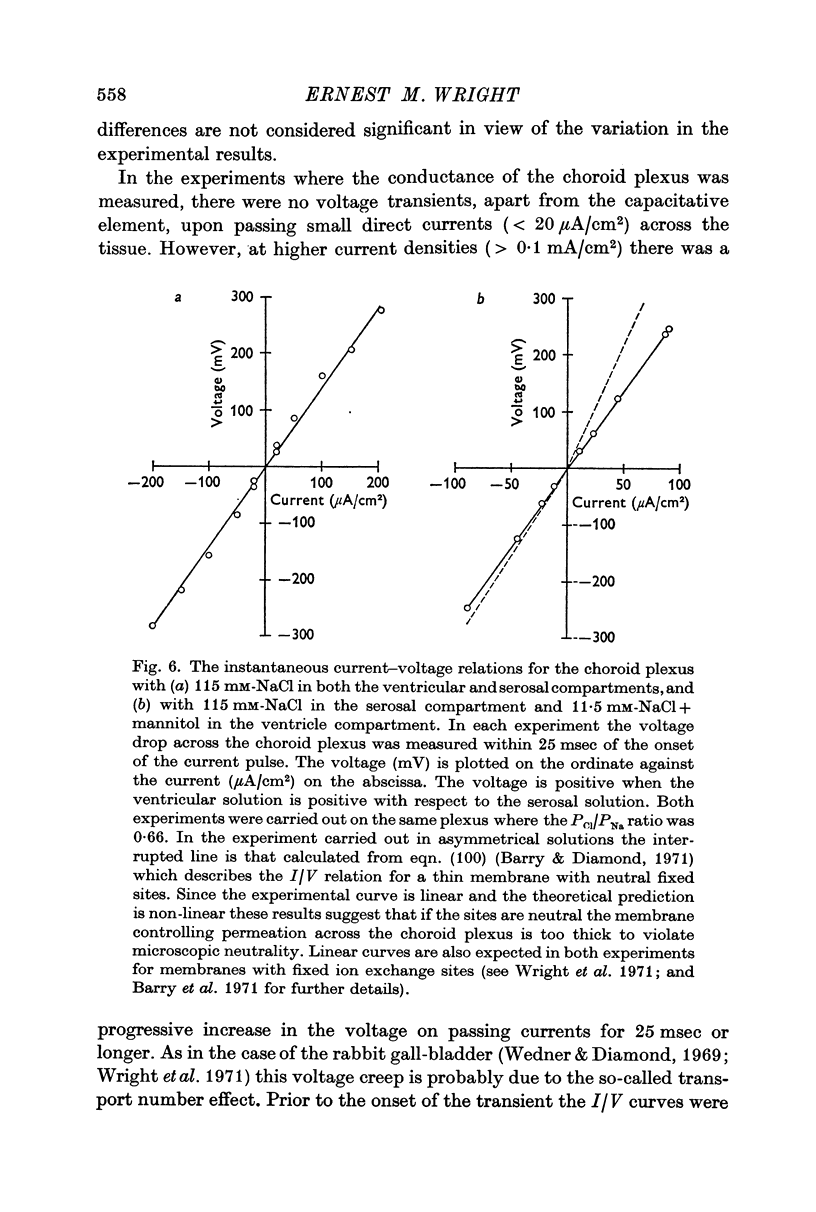
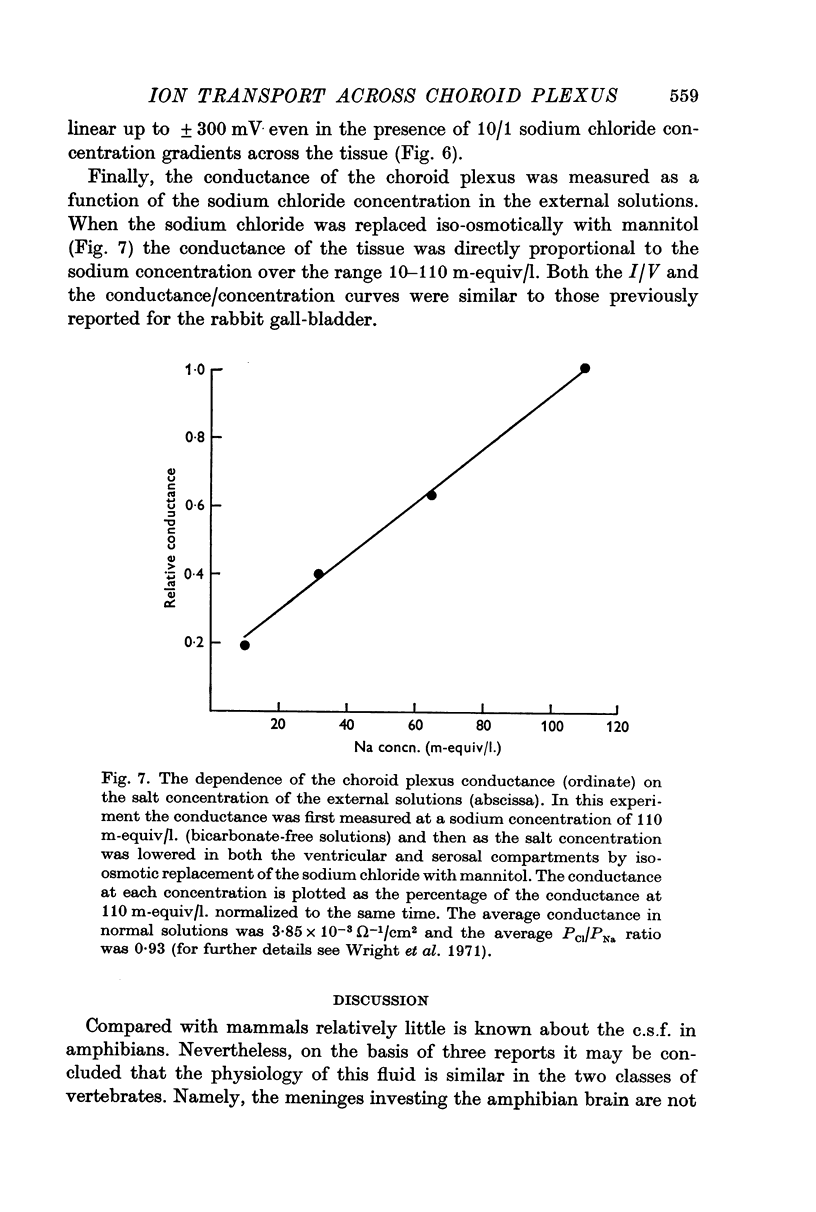
7. The instantaneous current—voltage curves were linear in both symmetrical and asymmetrical salt solutions and the choroid plexus conductance was found to be directly proportional to the external salt concentration. These and other lines of evidence suggest that the major route of passive ion permeation across this epithelium is via the tight junction route and not through the cell interior.
8. These results are discussed in relation to the in vivo studies of c.s.f. secretion and the mechanisms of active and passive ion transport across other epithelial membranes such as the gall-bladder, intestine and renal proximal tubule.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Higashi K., Nesbett F. B. Effects of Pco2 acetazolamide and ouabain on volume and composition of choroid-plexus fluid. J Physiol. 1965 Dec;181(3):516–524. doi: 10.1113/jphysiol.1965.sp007780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd, Higashi K., Nesbett F. B. Relation of potassium concentration in choroidplexus fluid to that in plasma. J Physiol. 1965 Dec;181(3):506–515. doi: 10.1113/jphysiol.1965.sp007779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A., Segal M. B. Morphological changes associated with inhibition of fluid transport in the rabbit choroid plexus. J Physiol. 1970 Jun;208(2):88P–91P. [PubMed] [Google Scholar]
- Carpenter S. J. An electron microscopic study of the choroid plexuses of Necturus maculosus. J Comp Neurol. 1966 Jul;127(3):413–434. doi: 10.1002/cne.901270309. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Gerschenfeld H. M., Kuffler S. W. Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol. 1968 Jul;197(2):363–380. doi: 10.1113/jphysiol.1968.sp008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F. Physiology of the choroid plexus. Physiol Rev. 1971 Apr;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- Davson H., Segal M. B. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol. 1970 Jul;209(1):131–153. doi: 10.1113/jphysiol.1970.sp009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Functional consequences of ultrastructural geometry in "backwards" fluid-transporting epithelia. J Cell Biol. 1968 Jun;37(3):694–702. doi: 10.1083/jcb.37.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Domer F. R. Effects of diuretics on cerebrospinal fluid formation and potassium movement. Exp Neurol. 1969 May;24(1):54–64. doi: 10.1016/0014-4886(69)90005-3. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Schultz S. G. Ionic conductances of extracellular shunt pathway in rabbit ileum. Influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol. 1972 Mar;59(3):318–346. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Heisey S. R. Brain and choroid plexus blood volumes in vertebrates. Comp Biochem Physiol. 1968 Aug;26(2):489–498. doi: 10.1016/0010-406x(68)90641-5. [DOI] [PubMed] [Google Scholar]
- Hersey S. J., High W. L. On the mechanism of acid secretory inhibition by acetazolamide. Biochim Biophys Acta. 1971 Jun 1;233(3):604–609. doi: 10.1016/0005-2736(71)90159-3. [DOI] [PubMed] [Google Scholar]
- Leusen I. Regulation of cerebrospinal fluid composition with reference to breathing. Physiol Rev. 1972 Jan;52(1):1–56. doi: 10.1152/physrev.1972.52.1.1. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H., Sugioka K. pH of cerebrospinal fluid in the cisterna Magna and on the surface of the choroid plexus of the 4th ventricle and its effect on ventilation in experimental disturbances of acid base balance. Transients and steady states. Pflugers Arch. 1969;312(4):161–188. doi: 10.1007/BF00586927. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Diamond J. M. The mechanism of anion permeation in thorium-treated gallbladder. J Membr Biol. 1972;8(1):63–96. doi: 10.1007/BF01868095. [DOI] [PubMed] [Google Scholar]
- Macri F. J., Politoff A., Rubin R., Dixon R., Rall D. Preferential vasoconstrictor properties of acetazolamide on the arteries of the choroid plexus. Int J Neuropharmacol. 1966 Jan;5(1):109–115. doi: 10.1016/0028-3908(66)90056-6. [DOI] [PubMed] [Google Scholar]
- Oschman J. L., Berridge M. J. The structural basis of fluid secretion. Fed Proc. 1971 Jan-Feb;30(1):49–56. [PubMed] [Google Scholar]
- Schilb T. P., Brodsky W. A. CO 2 gradients and acidification by transport of HCO 3 in turtle bladders. Am J Physiol. 1972 Feb;222(2):272–281. doi: 10.1152/ajplegacy.1972.222.2.272. [DOI] [PubMed] [Google Scholar]
- Schulz I., Ströver F., Ullrich K. J. Lipid soluble weak organic acid buffers as "substrate" for pancreatic secretion. Pflugers Arch. 1971;323(2):121–140. doi: 10.1007/BF00586444. [DOI] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Fordtran J. S., Carter N. W., Rector F. C., Jr Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Invest. 1970 Mar;49(3):548–556. doi: 10.1172/JCI106265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Radtke H. W., Rumrich G. The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflugers Arch. 1971;330(2):149–161. doi: 10.1007/BF00643031. [DOI] [PubMed] [Google Scholar]
- VATES T. S., Jr, BONTING S. L., OPPELT W. W. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE FORMATION OF CEREBROSPINAL FLUID IN THE CAT. Am J Physiol. 1964 May;206:1165–1172. doi: 10.1152/ajplegacy.1964.206.5.1165. [DOI] [PubMed] [Google Scholar]
- Welch K. The secretion of cerebrospinal fluid by lamina epithelialis. Monogr Surg Sci. 1967 Sep;4(3):155–192. [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Anion transport by isolated rabbit gall bladders. Am J Physiol. 1967 Nov;213(5):1199–1204. doi: 10.1152/ajplegacy.1967.213.5.1199. [DOI] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Hydrogen ion transport by isolated rabbit gallbladder. Am J Physiol. 1969 Jul;217(1):310–316. doi: 10.1152/ajplegacy.1969.217.1.310. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus V. D., Sung C. P., Helander H. F., Shah G., Blum A. L., Sachs G. Solubilization of anion ATPase from necturus oxyntic cells. Biochim Biophys Acta. 1971 Jul 6;241(1):49–56. doi: 10.1016/0005-2736(71)90302-6. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Ion transport across the frog posterior choroid plexus. Brain Res. 1970 Oct 13;23(2):302–304. doi: 10.1016/0006-8993(70)90057-0. [DOI] [PubMed] [Google Scholar]


