Abstract
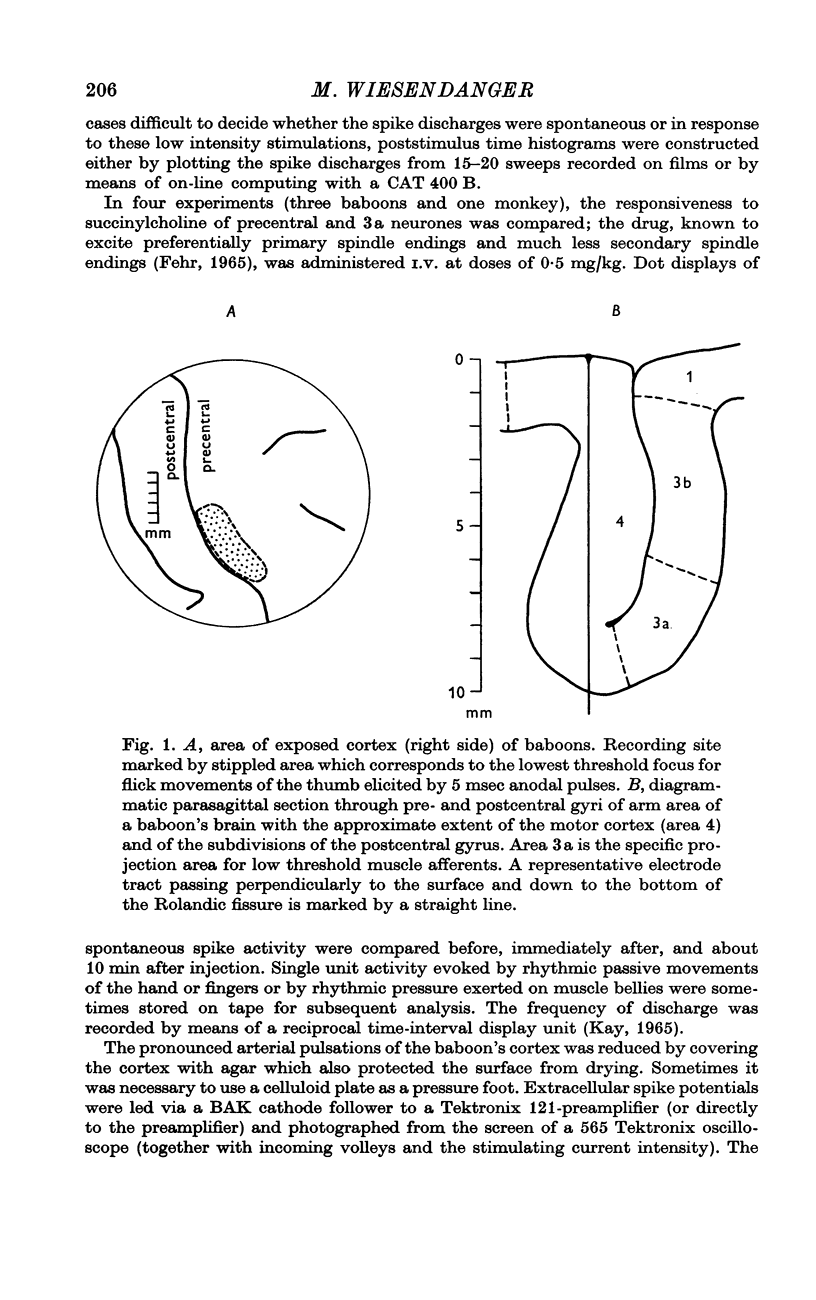
1. The precentral bank of the Rolandic fissure of the cortical arm area has been explored with extracellular micro-electrodes in primates (baboons and monkeys) under nitrous oxide and oxygen anaesthesia, supplemented by small doses of Parkesernyl® and chloralose. The results in baboons and monkeys were the same.
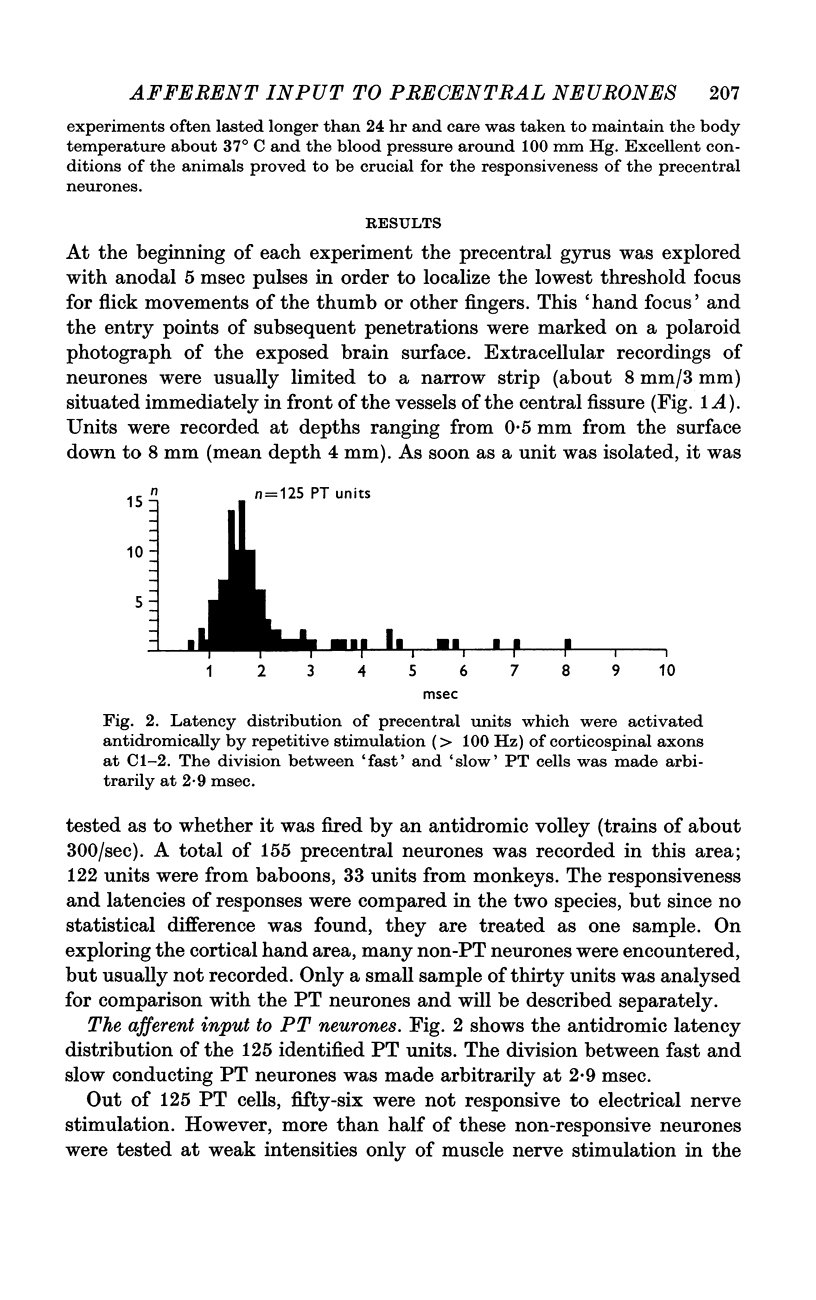
2. Single units were classified as pyramidal tract neurones or non-pyramidal tract neurones according to their antidromic responsiveness to stimuli applied in the dorsolateral funiculus at C1-2.
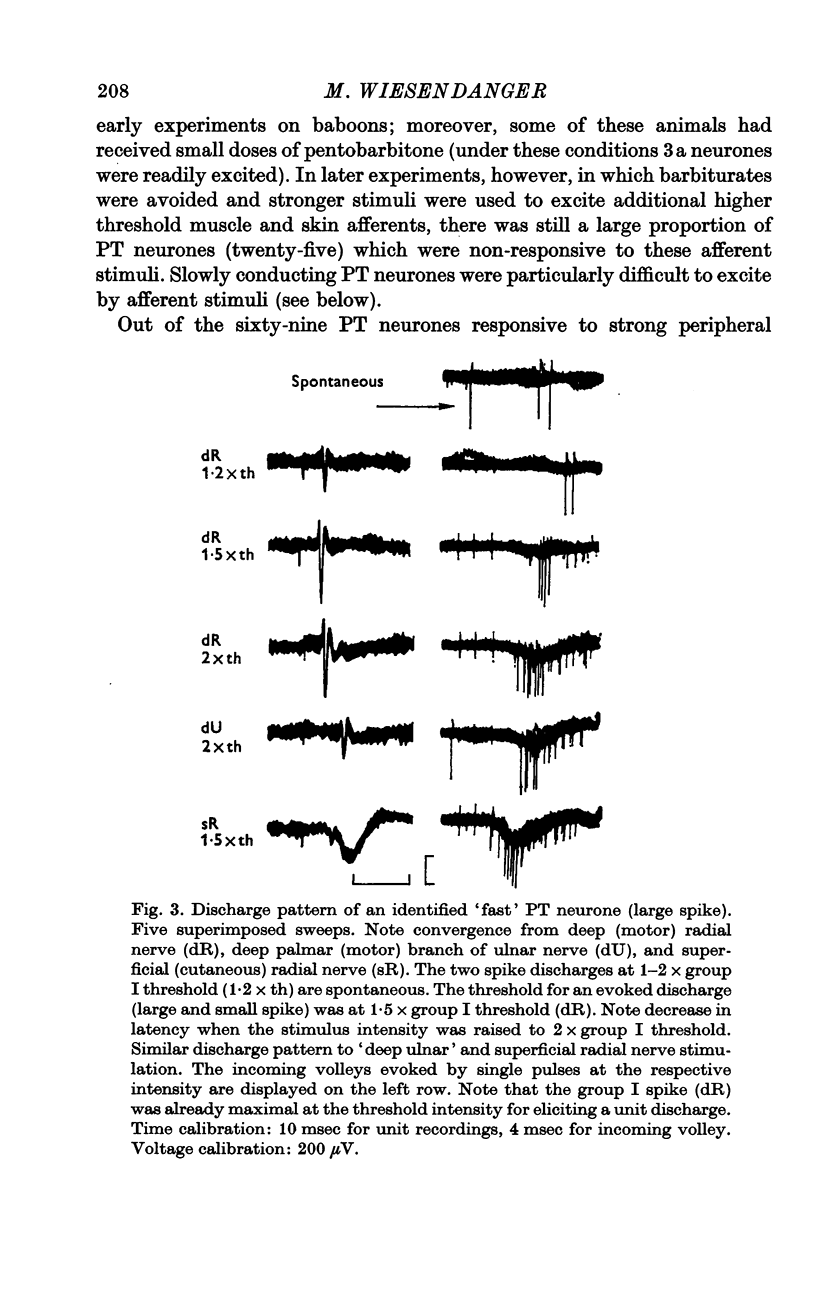
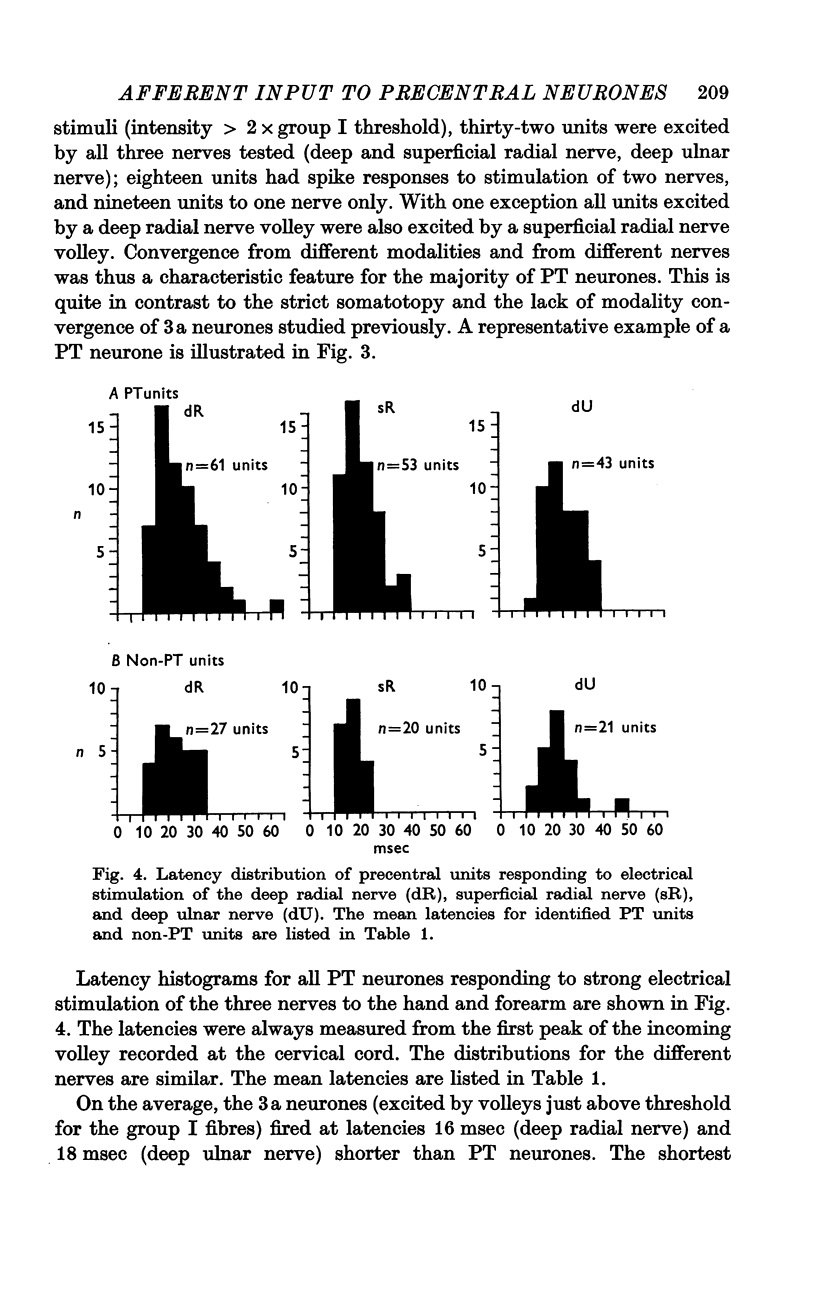
3. Responses to electrical stimulation of the deep (motor) radial nerve, the deep palmar (motor) branch of the ulnar nerve, and the superficial (cutaneous) radial nerve could be recorded in the majority of neurones of the motor cortex provided that short trains of strong stimuli were used. Minimal responses to muscle nerve stimulation were observed in a few neurones at 1·4 × group I threshold, but most units reacted only with higher stimulus intensities (2-3 × group I threshold).
4. The latencies to peripheral nerve stimulation were measured from the first peak of the incoming volley recorded at the root entry zone. The mean response latencies of pyramidal tract cells were between 20 and 25 msec; non-pyramidal tract cells were activated at slightly shorter mean latencies, the difference being significant for superficial radial nerve stimulation only (4 msec). These latencies are more than twice as long as those recorded in the postcentral gyrus, and the probability of discharge is lower than for postcentral neurones.
5. A further difference between neurones of the postcentral and precentral gyrus is the pronounced convergence from different nerves and also from different modalities (cutaneous and muscle afferents) in units of the precentral cortex in contrast to units of the postcentral cortex.
6. The high thresholds, necessary to activate precentral neurones by muscle nerve stimulation, make it unlikely that group I muscle afferents are involved. This is, furthermore, indicated by the lack of responsiveness to intravenous injection of succinylcholine which was, however, effective for driving neurones of the specific projection area for group I afferents, area 3a. The present experiments are consistent with the view that sensitivity of precentral neurones to muscle stretch (described in previous studies) is due to activation of secondary muscle spindle endings and their ascending pathways.
7. The original hypothesis of a load compensating `pyramidal reflex' with an oligosynaptic afferent contribution from the spindle primaries can be discarded. The present findings indicate that there is a feed-back from secondary muscle spindle afferents which, by way of a more complex pathway, can modulate the firing frequency of neurones in the motor cortex.
Full text
PDF



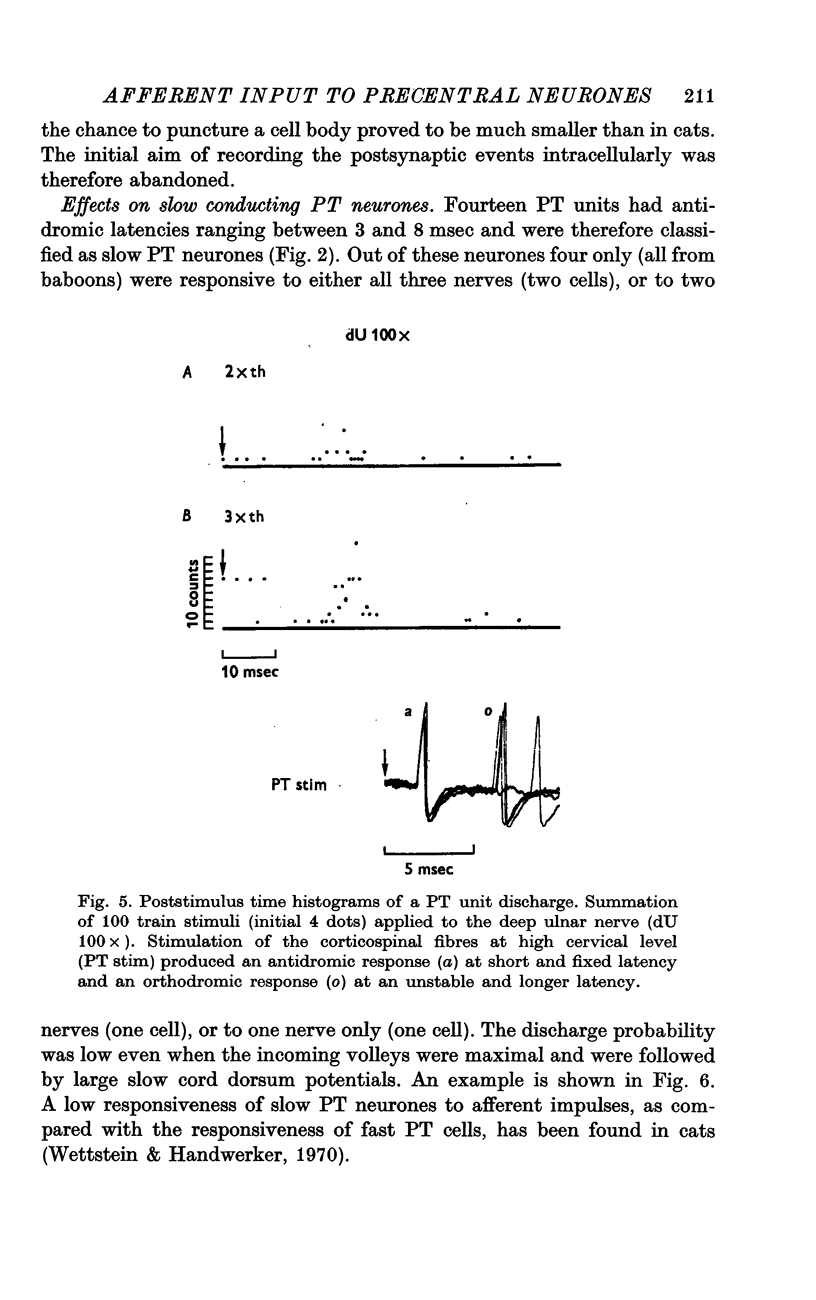
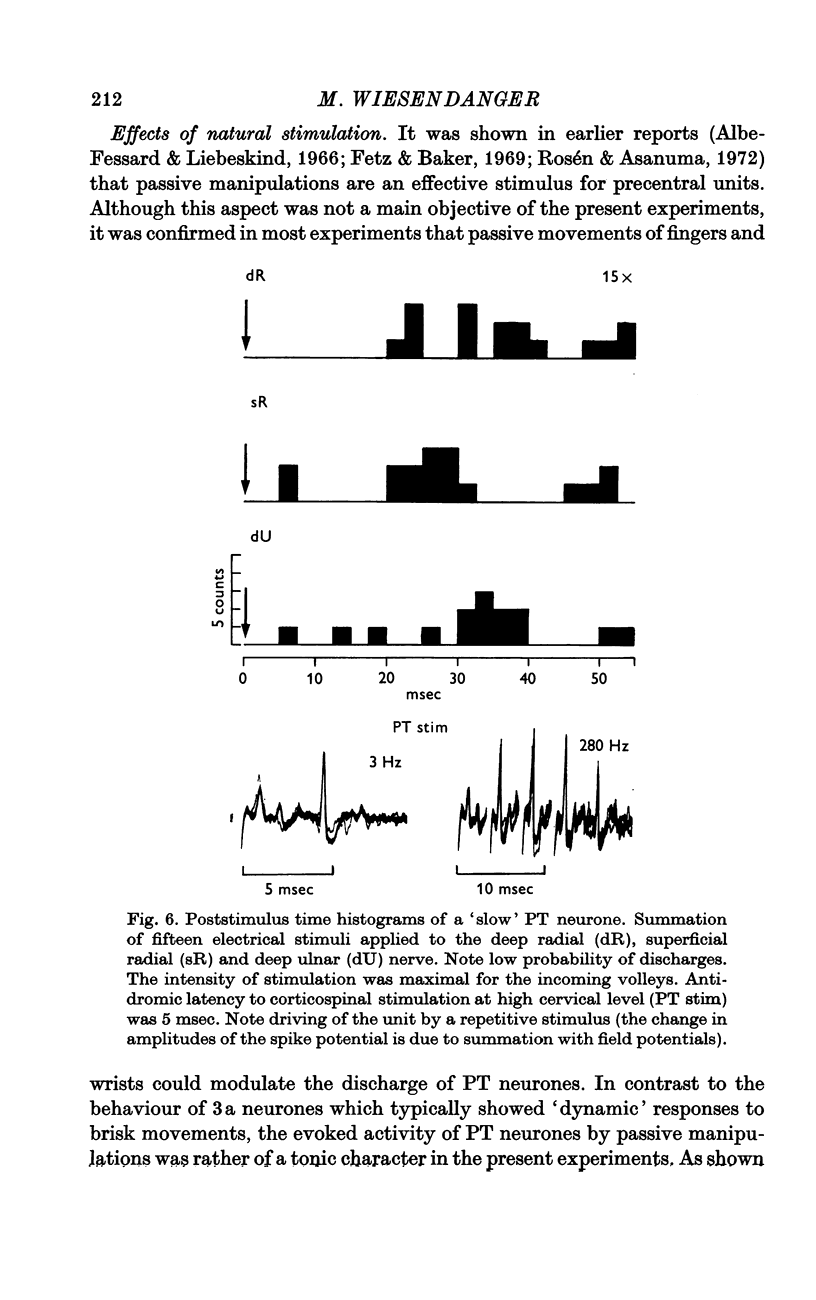
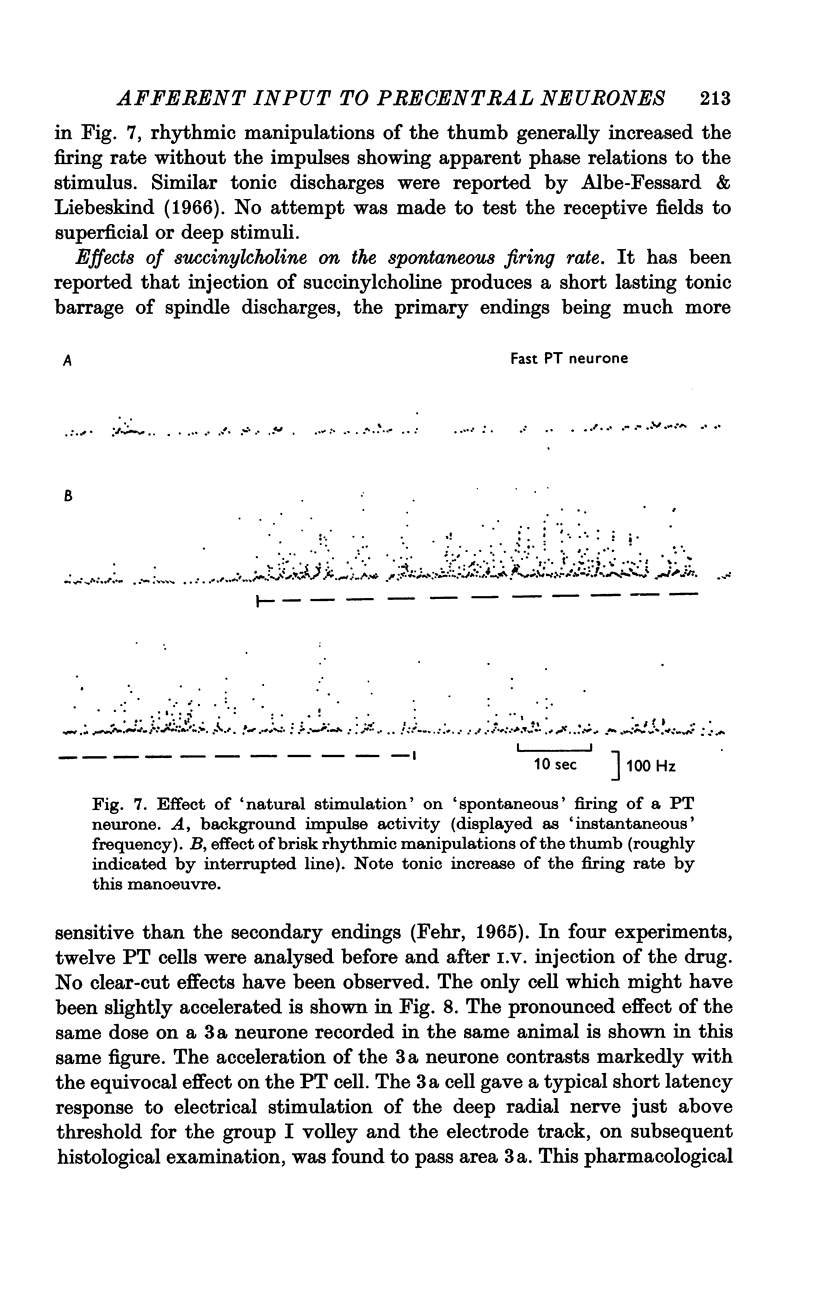
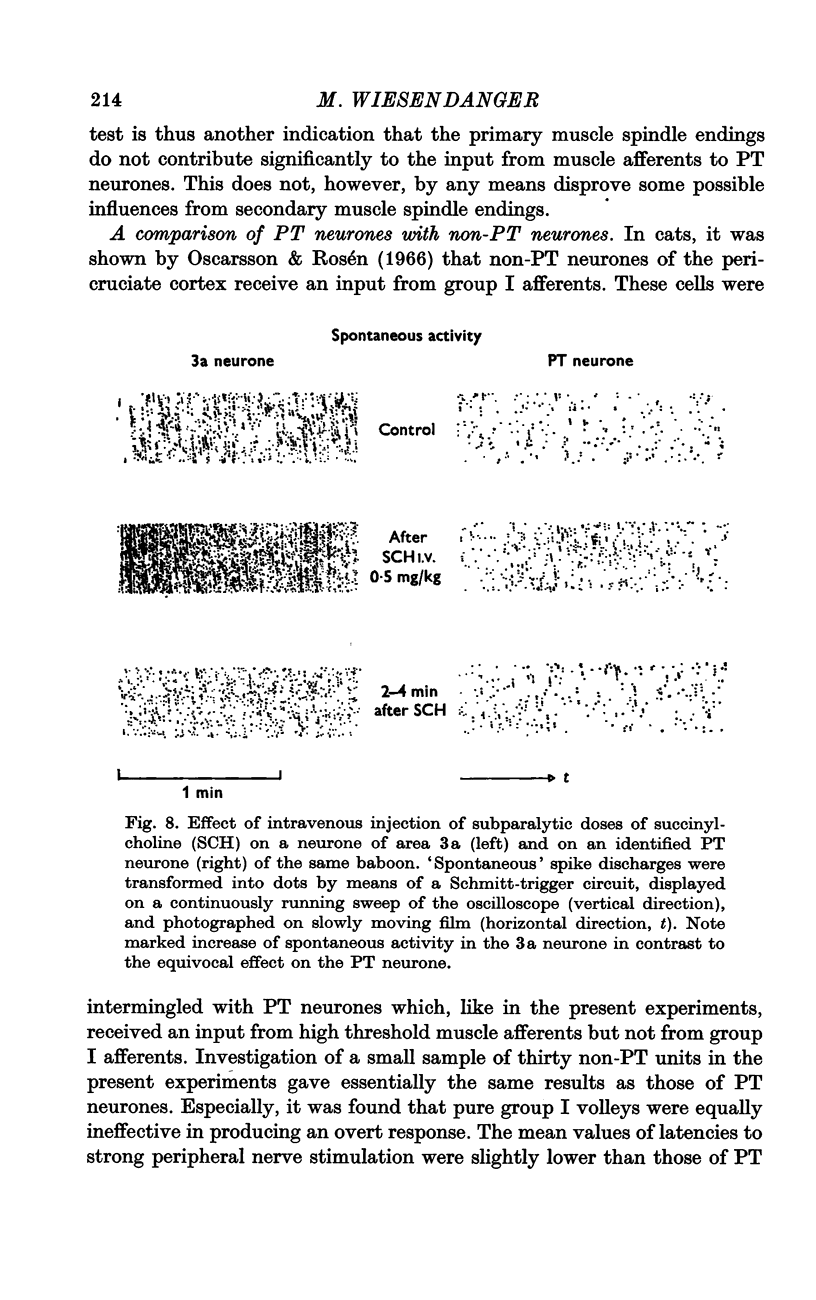





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albe-Fessard D., Liebeskind J. Origine des messages somato-sensitifs activant les cellules du cortex moteur chez le singe. Exp Brain Res. 1966;1(2):127–146. doi: 10.1007/BF00236866. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14(3):243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Doetsch G. S., Gardner E. B. Relationship between afferent input and motor output in sensorimotor cortex of the monkey. Exp Neurol. 1972 Apr;35(1):78–97. doi: 10.1016/0014-4886(72)90061-1. [DOI] [PubMed] [Google Scholar]
- FEHR H. U. ACTIVATION BY SUXAMETHONIUM OF PRIMARY AND SECONDARY ENDINGS OF THE SAME DE-EFFERENTED MUSCLE SPINDLE DURING STATIC STRETCH. J Physiol. 1965 May;178:98–110. doi: 10.1113/jphysiol.1965.sp007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S., Ratcheson R. Human motor cortex: sensory input data from single neuron recordings. Science. 1972 Mar 31;175(4029):1493–1495. doi: 10.1126/science.175.4029.1493. [DOI] [PubMed] [Google Scholar]
- Gordon G., Miller R. Identification of cortical cells projecting to the dorsal column nuclei of the cat. Q J Exp Physiol Cogn Med Sci. 1969 Jan;54(1):85–98. doi: 10.1113/expphysiol.1969.sp002009. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. F., COXE W. S. Representation of cutaneous tactile sensibility in cerebral cortex of Cebus. J Neurophysiol. 1958 Sep;21(5):481–498. doi: 10.1152/jn.1958.21.5.481. [DOI] [PubMed] [Google Scholar]
- KRUGER L. Characteristics of the somatic afferent projection to the precentral cortex in the monkey. Am J Physiol. 1956 Sep;186(3):475–482. doi: 10.1152/ajplegacy.1956.186.3.475. [DOI] [PubMed] [Google Scholar]
- Konorski J. The problem of the peripheral control of skilled movements. Int J Neurosci. 1970 Oct;1(1):39–50. doi: 10.3109/00207457009147616. [DOI] [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. PRESYNAPTIC HYPERPOLARIZATION: A ROLE FOR FINE AFFERENT FIBRES. J Physiol. 1964 Aug;172:274–294. doi: 10.1113/jphysiol.1964.sp007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi M. Differential block of conduction of larger fibers in peripheral nerve by direct current. Arch Ital Biol. 1970 Jan;108(1):52–71. [PubMed] [Google Scholar]
- Oscarsson O., Rosén I. Short-latency projections to the cat's cerebral cortex from skin and muscle afferents in the contralateral forelimb. J Physiol. 1966 Jan;182(1):164–184. doi: 10.1113/jphysiol.1966.sp007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. G., Powell T. P., Wiesendanger M. Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon's cortex. J Physiol. 1971 Sep;217(2):419–446. doi: 10.1113/jphysiol.1971.sp009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén I., Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Exp Brain Res. 1972;14(3):257–273. doi: 10.1007/BF00816162. [DOI] [PubMed] [Google Scholar]
- Wettstein A., Handwerker H. O. Afferente Verbindungen zu schnell- und langsamleitenden Pyramidenbahnneuronen der Katze. Pflugers Arch. 1970;320(3):247–260. doi: 10.1007/BF00587456. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. Effects of electrical stimulation of peripheral nerves to the hand and forearm on pyramidal tract neurones of the baboon and monkey. Brain Res. 1972 May 12;40(1):193–202. doi: 10.1016/0006-8993(72)90127-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman I. D. A triple representation of the body surface in the sensorimotor cortex of the squirrel monkey. Exp Neurol. 1968 Mar;20(3):415–431. doi: 10.1016/0014-4886(68)90084-8. [DOI] [PubMed] [Google Scholar]


