Abstract
1. In cats anaesthetized with Nembutal, the cutaneous receptive fields of individual cerebellar climbing fibres were assessed by recording the climbing fibre responses of single Purkyně cells following controlled mechanical stimulation (air jets, vibration, taps, pressure) of the foot pads of all four limbs and of the hairy skin of the limbs and the body.
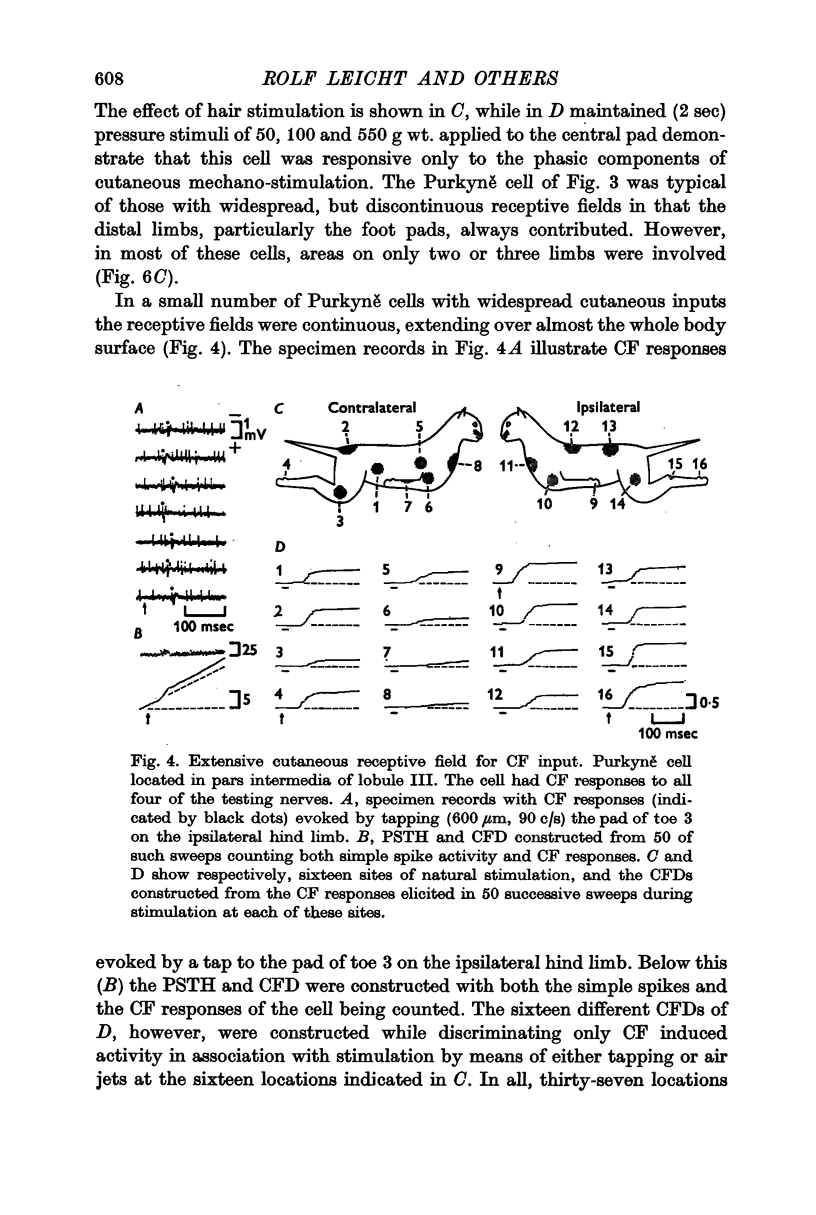
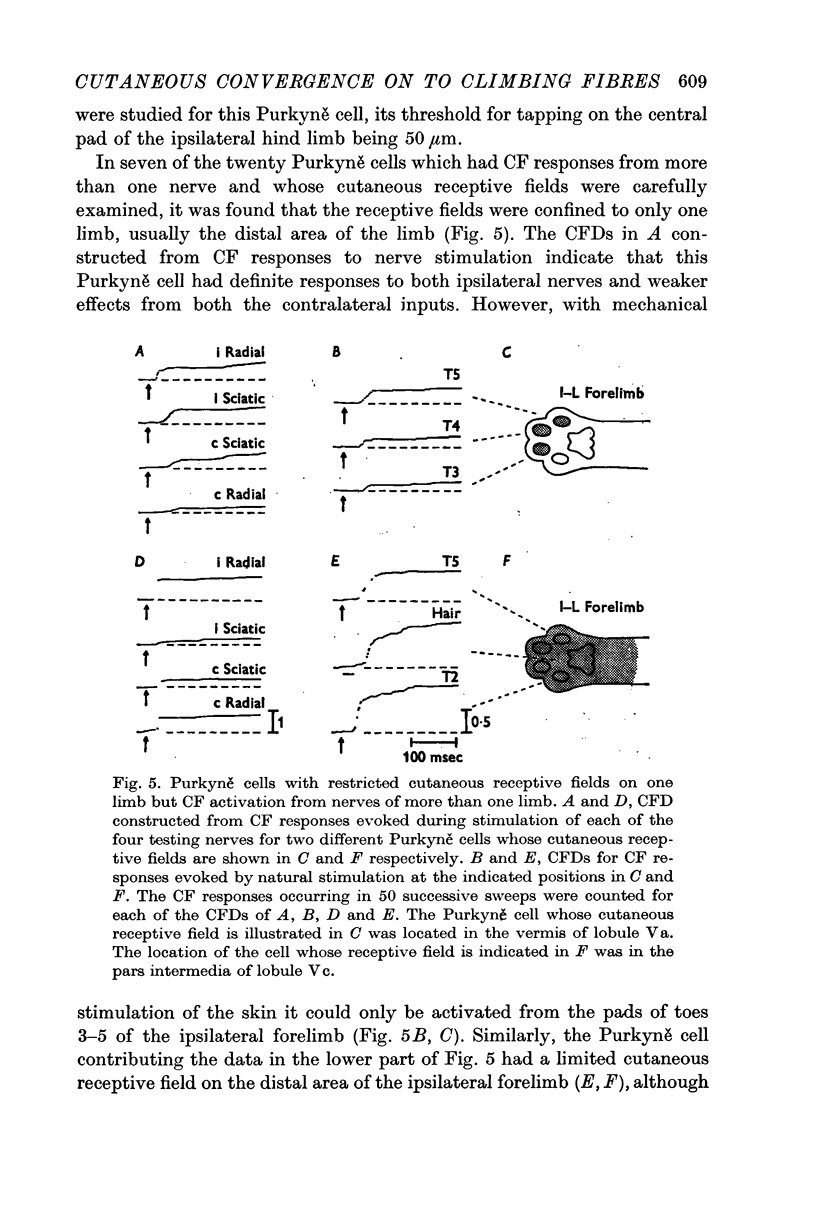
2. Three major types of cutaneous receptive fields of individual climbing fibres were recognized: (a) restricted fields generally confined to the distal areas of one limb only; (b) circumscribed fields on the distal areas of two to four limbs (discontinuous fields); and (c) widespread continuous fields extending over all or almost all of the body surface. A fourth group appears to receive cutaneous inputs from one limb and sensory input from deeper structures in other limbs.
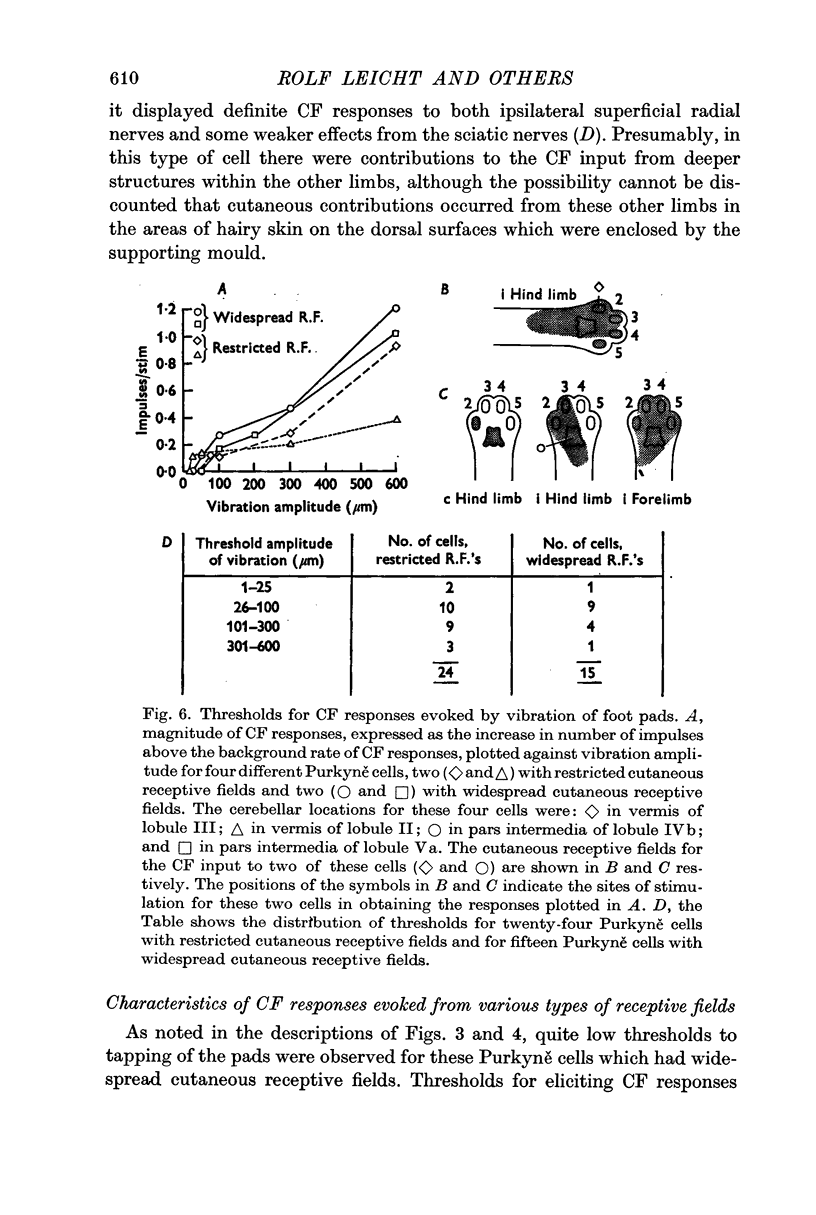
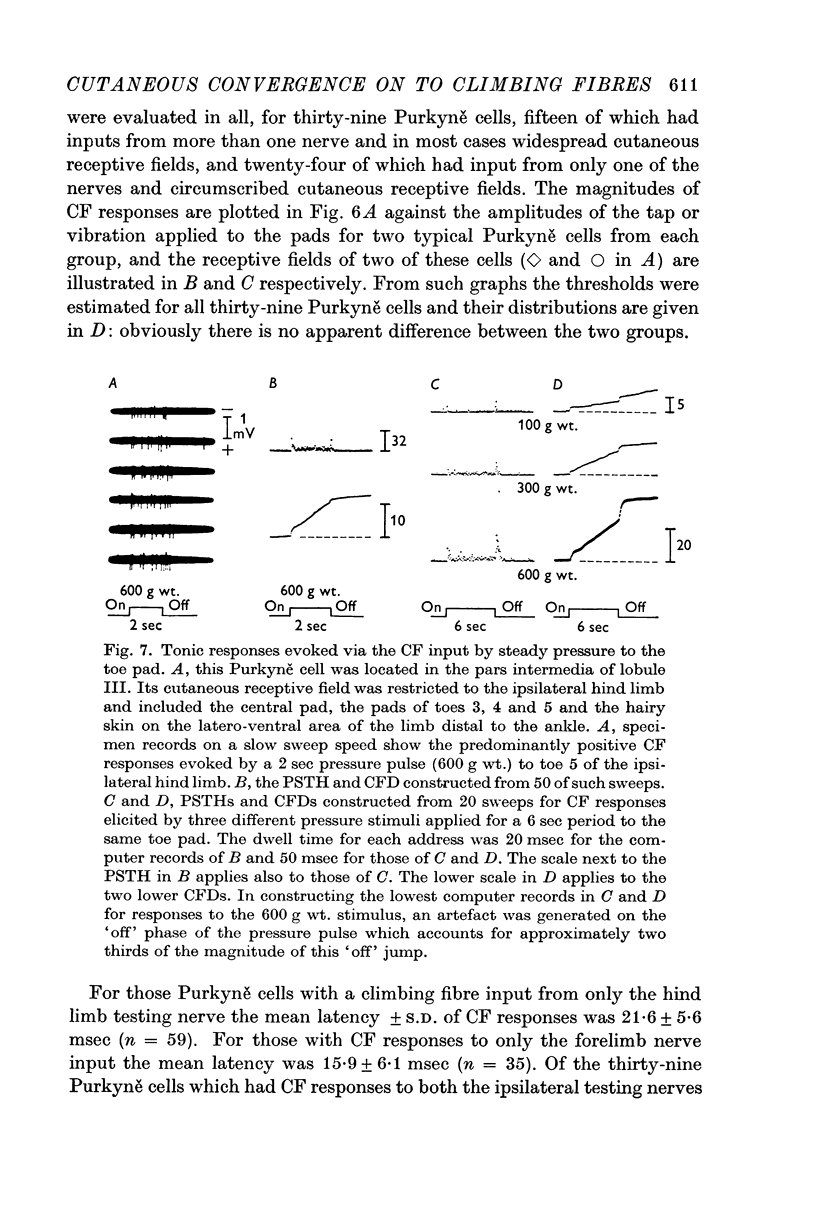
3. Thresholds for tapping of the foot pads were often quite low (< 100 μm indentation), and there was no noticeable difference in the distribution of thresholds between the climbing fibres having restricted or more widespread cutaneous receptive fields. Similarly, the latencies of climbing fibre responses evoked by vibration and tapping were in the same ranges for climbing fibres with receptive fields restricted to one limb only and for those having more widespread fields.
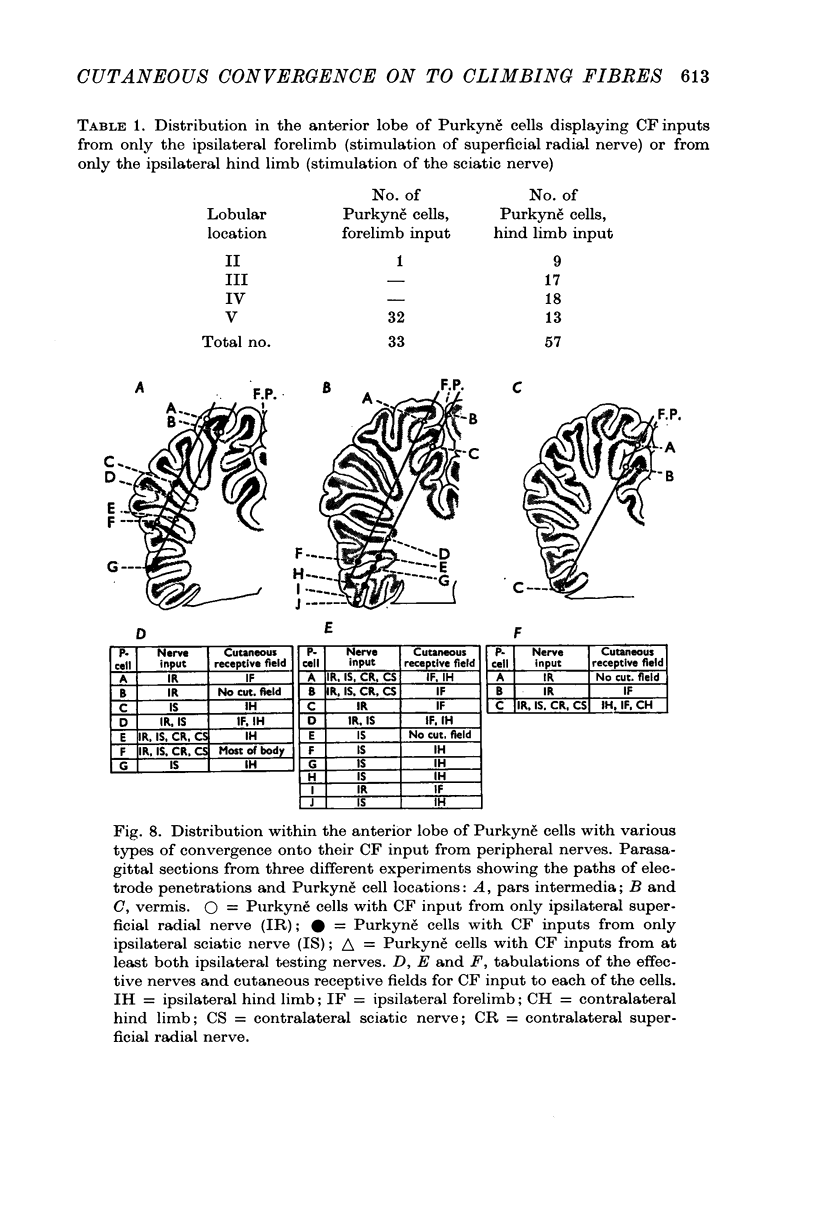
4. In regard to location in the anterior lobe of the cerebellum it was found that those climbing fibres receiving inputs only from the ipsilateral forelimb projected to Purkyně cells located almost entirely in lobule V, whereas all other climbing fibres with restricted or more widespread receptive fields projected to Purkyně cells distributed widely within lobules II to V.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkyne cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Investigations on integration of mossy fiber inputs to Purkyne cells in the anterior lobe. Exp Brain Res. 1971 Jul 26;13(1):54–77. [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Topographical investigations on the climbing fiber inputs from forelimb and hindlimb afferents to the cerebellar anterior lobe. Exp Brain Res. 1968;6(3):195–215. doi: 10.1007/BF00235124. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. 3. In Purkyne cells by climbing fiber input. Exp Brain Res. 1972 Oct 29;15(5):484–497. doi: 10.1007/BF00236404. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. I. In mossy fibers. Exp Brain Res. 1972;15(3):245–260. doi: 10.1007/BF00235910. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. II. In Purkyne cells by mossy fiber input. Exp Brain Res. 1972;15(3):261–277. doi: 10.1007/BF00235911. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Integration by Purkyne cells of mossy and climbing fiber inputs from cutaneous mechanoreceptors. Exp Brain Res. 1972 Oct 29;15(5):498–520. doi: 10.1007/BF00236405. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Kawaguchi S., Rowe M. J. Actions of afferent impulses from muscle receptors on cerebellar Purkyne cells. I. Responses to muscle vibration. Exp Brain Res. 1972;15(2):177–193. doi: 10.1007/BF00235581. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Leicht R., Rowe M. J., Schmidt R. F. Cortical and peripheral modification of cerebellar climbing fibre activity arising from cutaneous mechanoreceptors. J Physiol. 1973 Feb;228(3):619–635. doi: 10.1113/jphysiol.1973.sp010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini L., Redman S., Strata P. Mossy and climbing fibre organization on the anterior lobe of the cerebellum activated by forelimb and hindlimb areas of the sensorimotor cortex. Exp Brain Res. 1968;6(3):216–233. doi: 10.1007/BF00235125. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Senges J., Zimmermann M. Excitability measurements at the central terminals of single mechano-receptor afferents during slow potential changes. Exp Brain Res. 1967;3(3):220–233. doi: 10.1007/BF00235586. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F. Spinal cord afferents: functional organization and inhibitory control. UCLA Forum Med Sci. 1969;11:209–229. [PubMed] [Google Scholar]
- Sedgwick E. M., Williams T. D. Responses of single units in the inferior olive to stimulation of the limb nerves, peripheral skin receptors, cerebellum, caudate nucleus and motor cortex. J Physiol. 1967 Apr;189(2):261–279. doi: 10.1113/jphysiol.1967.sp008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]