Abstract
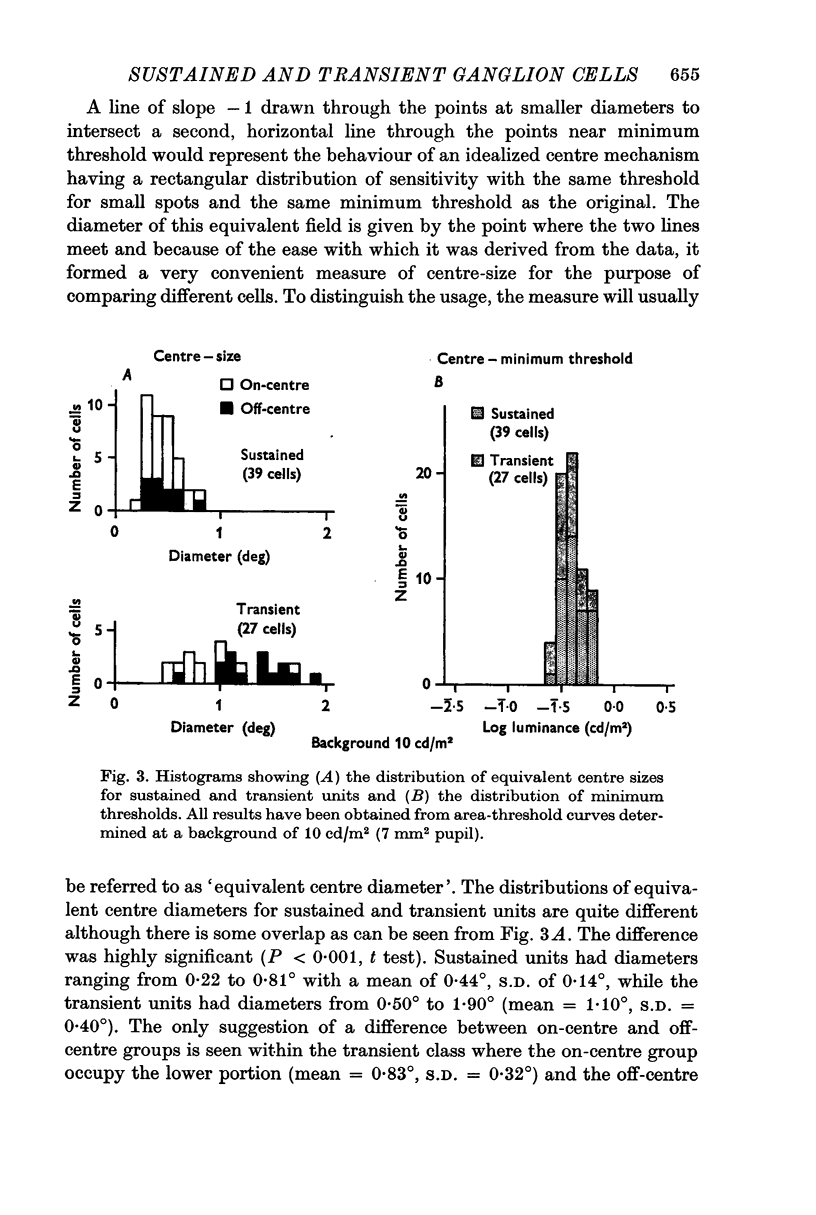
1. The functional basis for a sustained/transient classification of cat retinal ganglion cells has been strengthened by quantitative measurements of the sizes of the centre and surround components of receptive fields. Transient cells had larger surrounds than sustained; the distributions were non-overlapping. Although the distributions of centre sizes overlapped, the transient cells had very significantly larger centres on average.
2. There were characteristic differences in area-threshold and annulus-threshold curves and shapes of responses to brief and long flashes of light, and relative differences in the nature of surround adaptation and maintained discharge rates.
3. The distinction of sustained from transient units survived over a wide range of backgrounds including the two principle reorganizations of function: relative weakening of surround components at low background; Purkinjě shift at high.
4. Sustained and transient units were differentially distributed in the retina: there was an overwhelming preponderance of sustained units in the area centralis.
5. It is proposed that the transient units are the so-called multidendritedeep cells and the sustained units are the variously styled small ganglion cells.
Full text
PDF






























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., JEREMY D., LANCE J. W. The optic nerve; properties of a central tract. J Physiol. 1953 Aug;121(2):415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A. The nerve fibre composition of the cat optic nerve. J Anat. 1967 Jan;101(Pt 1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Honrubia F. M., Elliott J. H. Dendritic fields of the retinal ganglion cells in the cat. Arch Ophthalmol. 1970 Aug;84(2):221–226. doi: 10.1001/archopht.1970.00990040223016. [DOI] [PubMed] [Google Scholar]
- Honrubia F. M., Elliott J. H. Horizontal cell of the mammal retina. Arch Ophthalmol. 1969 Jul;82(1):98–104. doi: 10.1001/archopht.1969.00990020100024. [DOI] [PubMed] [Google Scholar]
- KIDD M. Electron microscopy of the inner plexiform layer of the retina in the cat and the pigeon. J Anat. 1962 Apr;96:179–187. [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- LEVICK W. R., OYSTER C. W., DAVIS D. L. EVIDENCE THAT MCILWAIN'S PERIPHERY EFFECT IS NOT A STRAY LIGHT ARTIFACT. J Neurophysiol. 1965 May;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- Leicester J., Stone J. Ganglion, amacrine and horizontal cells of the cat's retina. Vision Res. 1967 Sep;7(9):695–705. doi: 10.1016/0042-6989(67)90033-8. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Zacks J. L. Responses of cat retinal ganglion cells to brief flashes of light. J Physiol. 1970 Mar;206(3):677–700. doi: 10.1113/jphysiol.1970.sp009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Cervetto L. Homeostasis in retinal receptive fields. J Neurophysiol. 1971 Jul;34(4):579–587. doi: 10.1152/jn.1971.34.4.579. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. H. The structure responsible for action potential spikes in the cat's retina. Nature. 1949 Oct 29;164(4174):743–743. doi: 10.1038/164743a0. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Saito H. A., Shimahara T., Fukada Y. Four types of responses to light and dark spot stimuli in the cat optic nerve. Tohoku J Exp Med. 1970 Oct;102(2):127–133. doi: 10.1620/tjem.102.127. [DOI] [PubMed] [Google Scholar]
- Shkolnik-Yarros E. G. Neurons of the cat's retina. Vision Res. 1971 Jan;11(1):7–26. doi: 10.1016/0042-6989(71)90202-1. [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965 Jun;124(3):337–352. doi: 10.1002/cne.901240305. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Specialized receptive fields of the cat's retina. Science. 1966 May 27;152(3726):1277–1279. doi: 10.1126/science.152.3726.1277. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- Venes J. L., Collins W. F., Taub A. Nitrous oxide: an anesthetic for experiments in cats. Am J Physiol. 1971 Jun;220(6):2028–2031. doi: 10.1152/ajplegacy.1971.220.6.2028. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


