Abstract
1. The passive electrical properties of the membrane of the gastrooesophageal giant neurone (G cell) of the marine mollusc, Anisodoris nobilis were studied with small current steps.
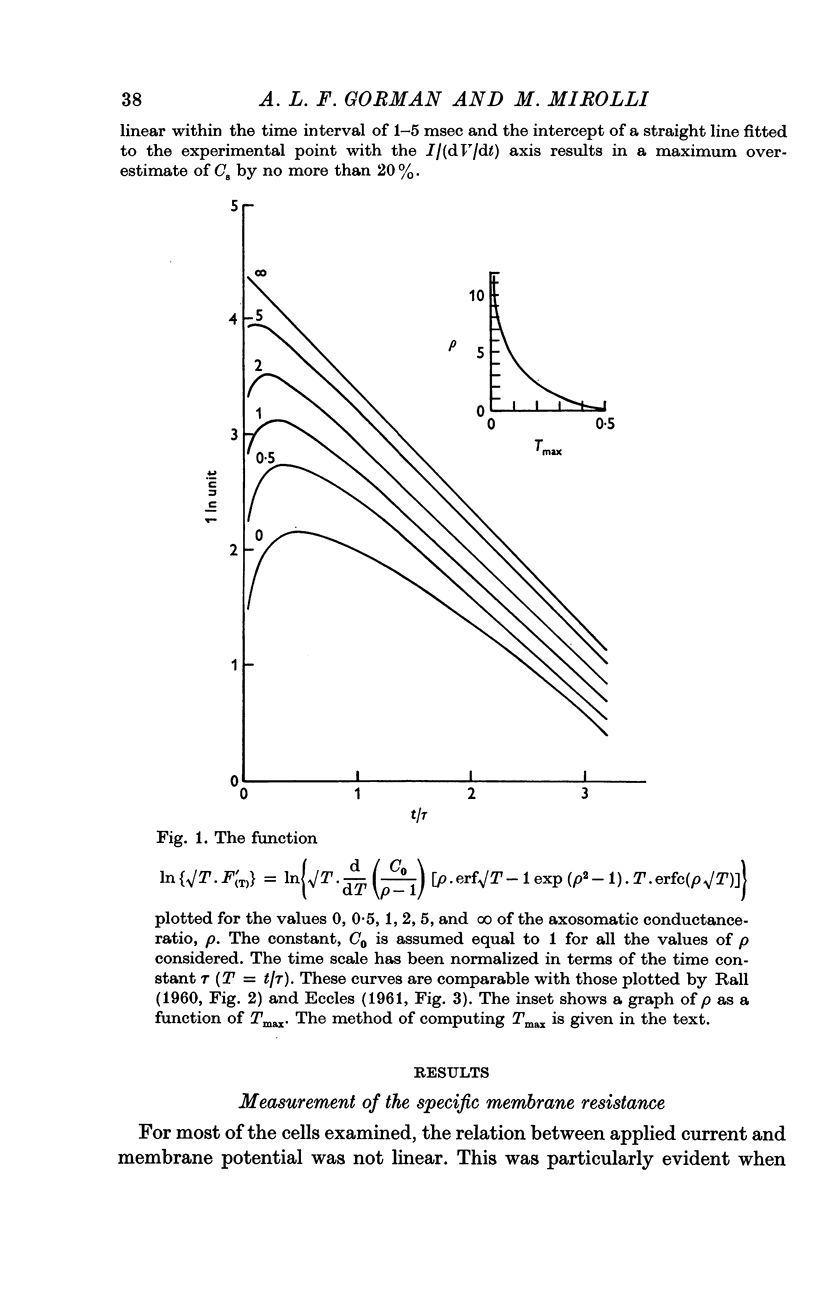
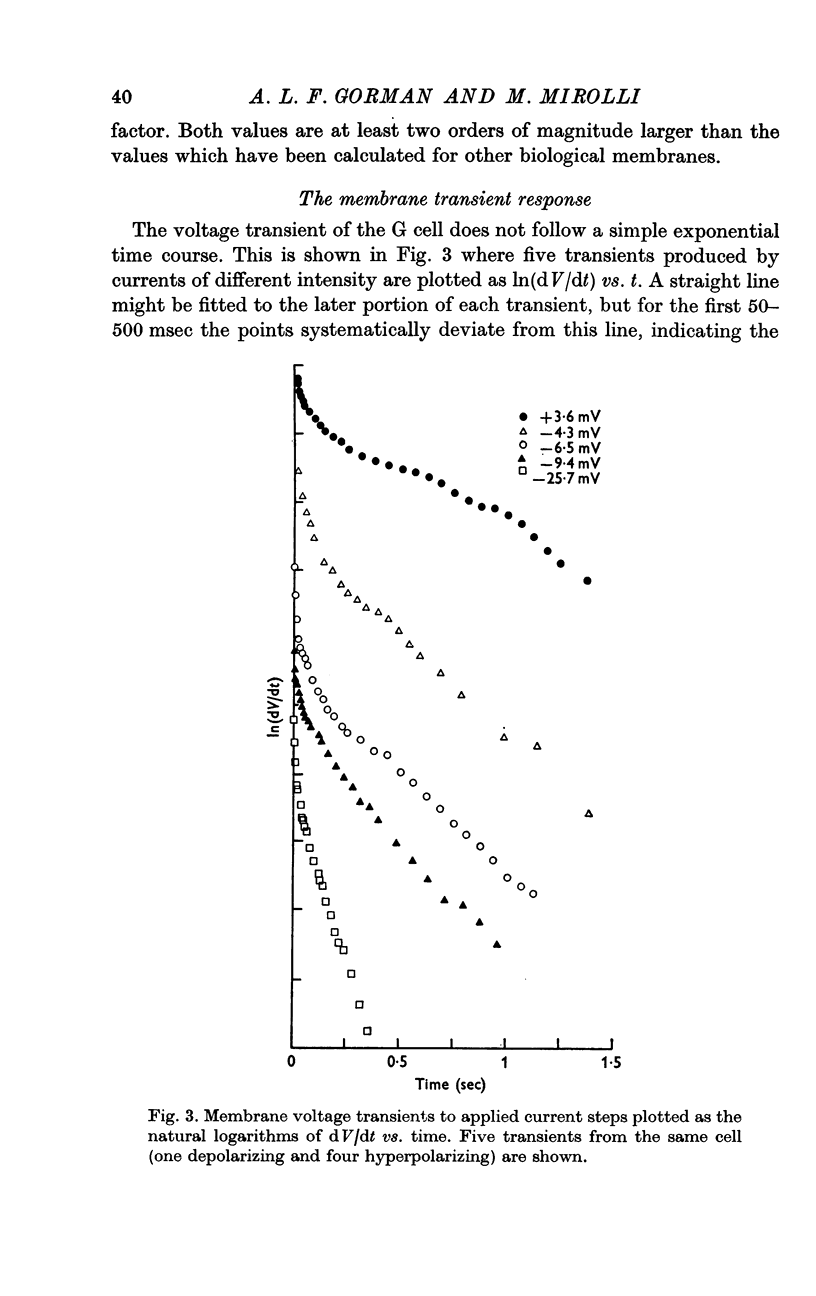
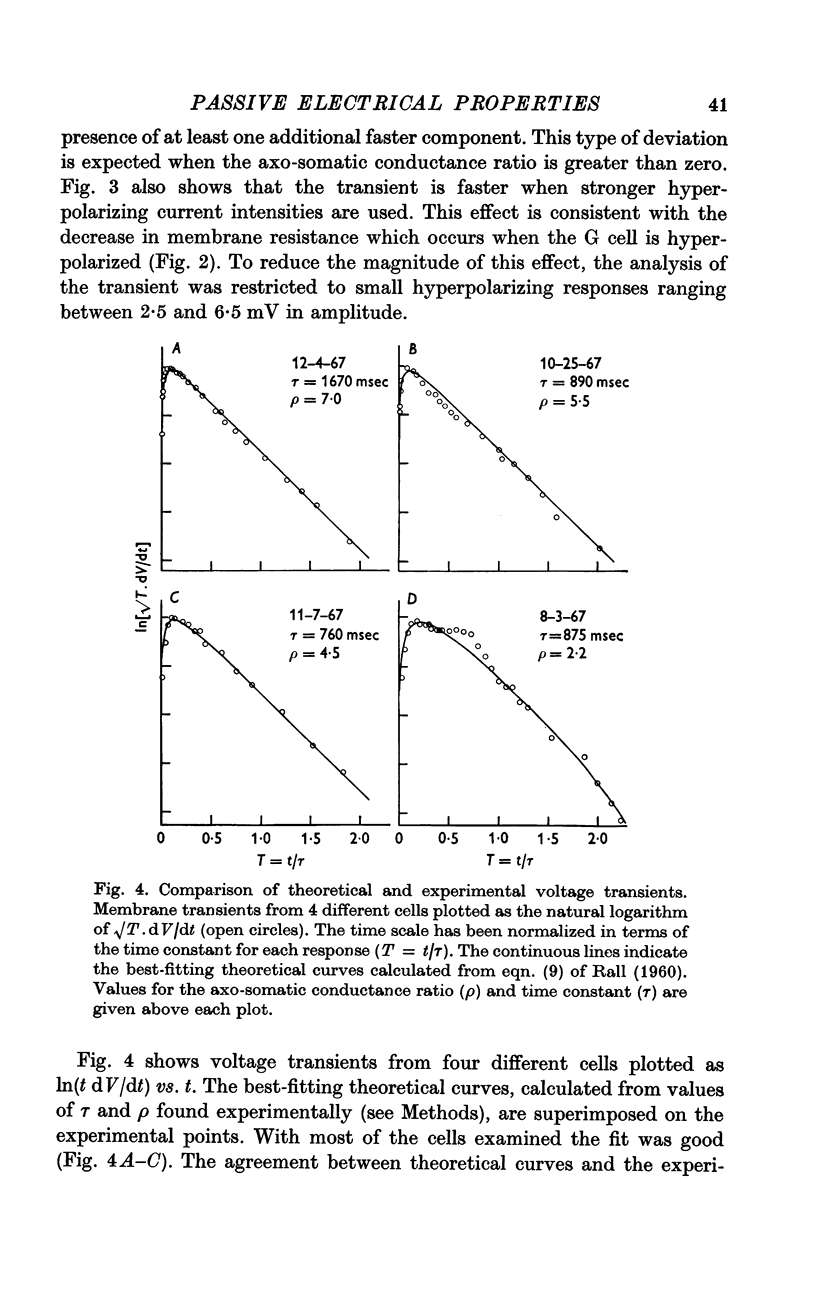
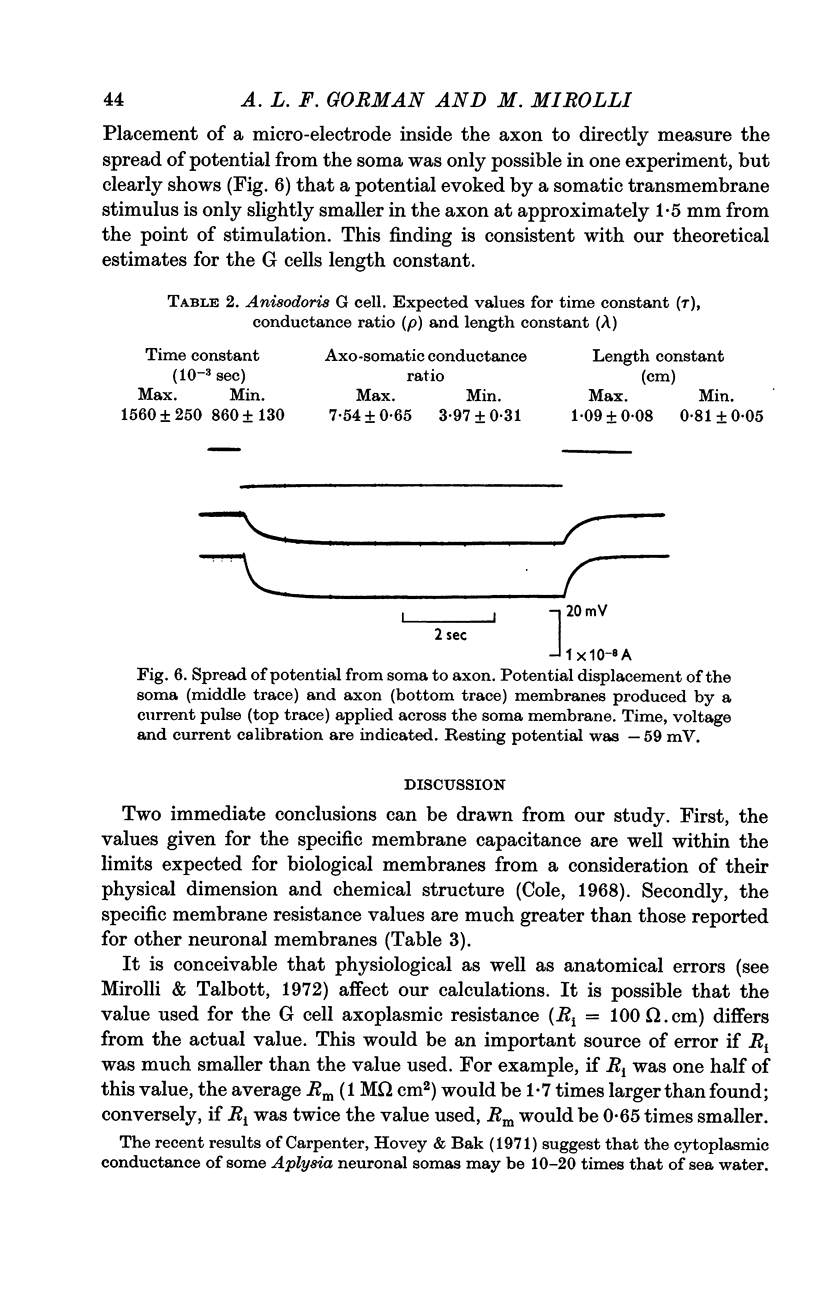
2. The membrane transient response can be fitted with a theoretical curve assuming as a model for the cell a sphere (soma) connected to a cable (axon). The axo-somatic conductance ratio (ρ), determined by applying this model, is large (approximately 5) and the membrane time constant (τ) is long (approximately 1 sec).
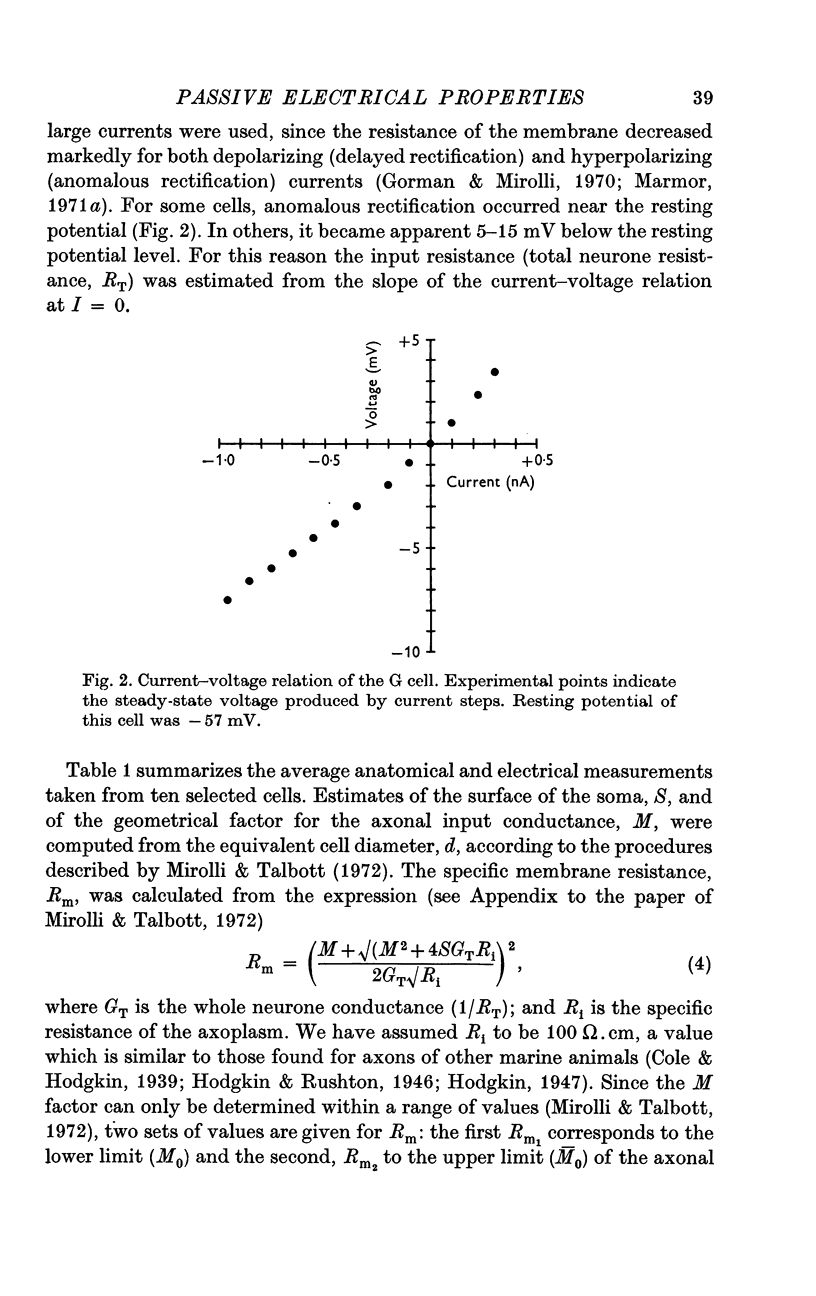
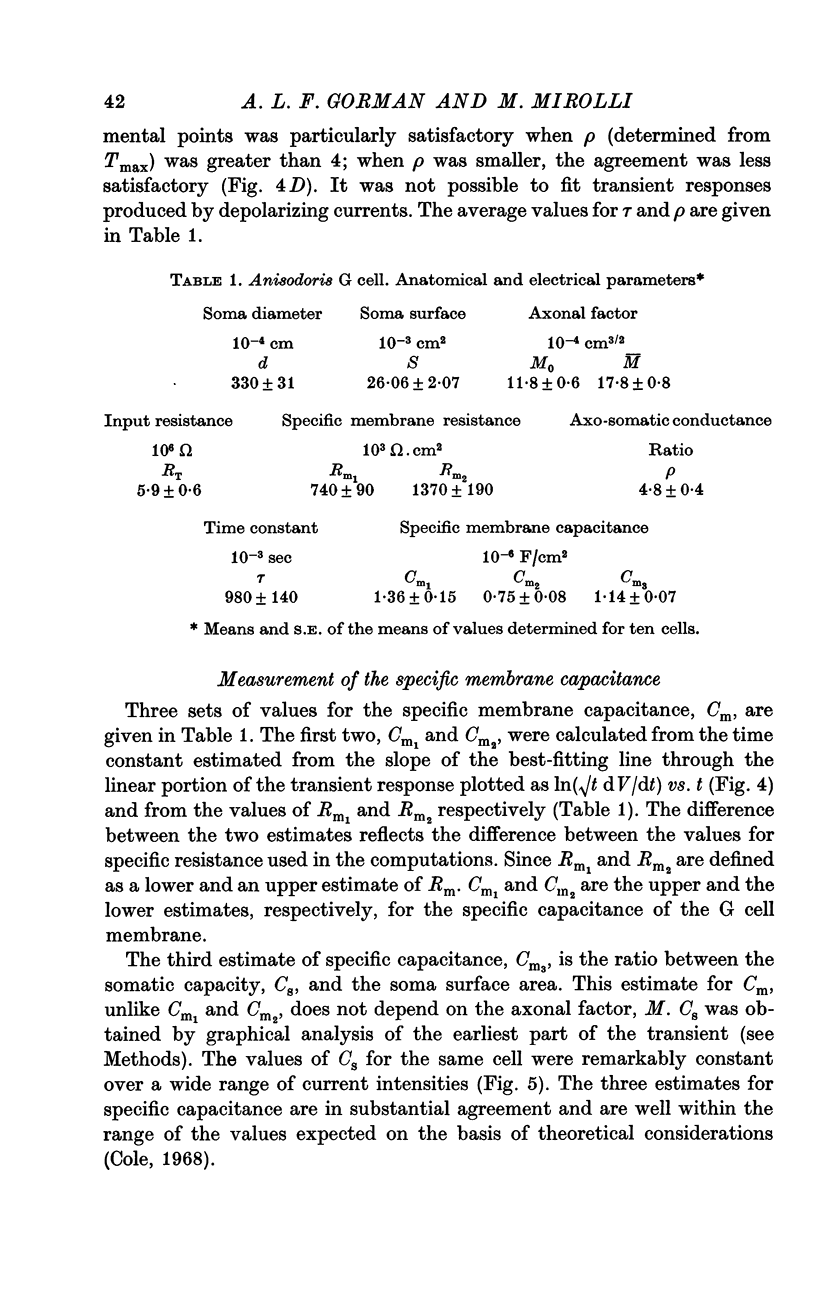
3. When the actual surface area of the cell, corrected for surface infoldings, and the spread of current along its axon is taken into account, the electrical measurements imply a specific resistance of the membrane of approximately 1·0 MΩ.cm2.
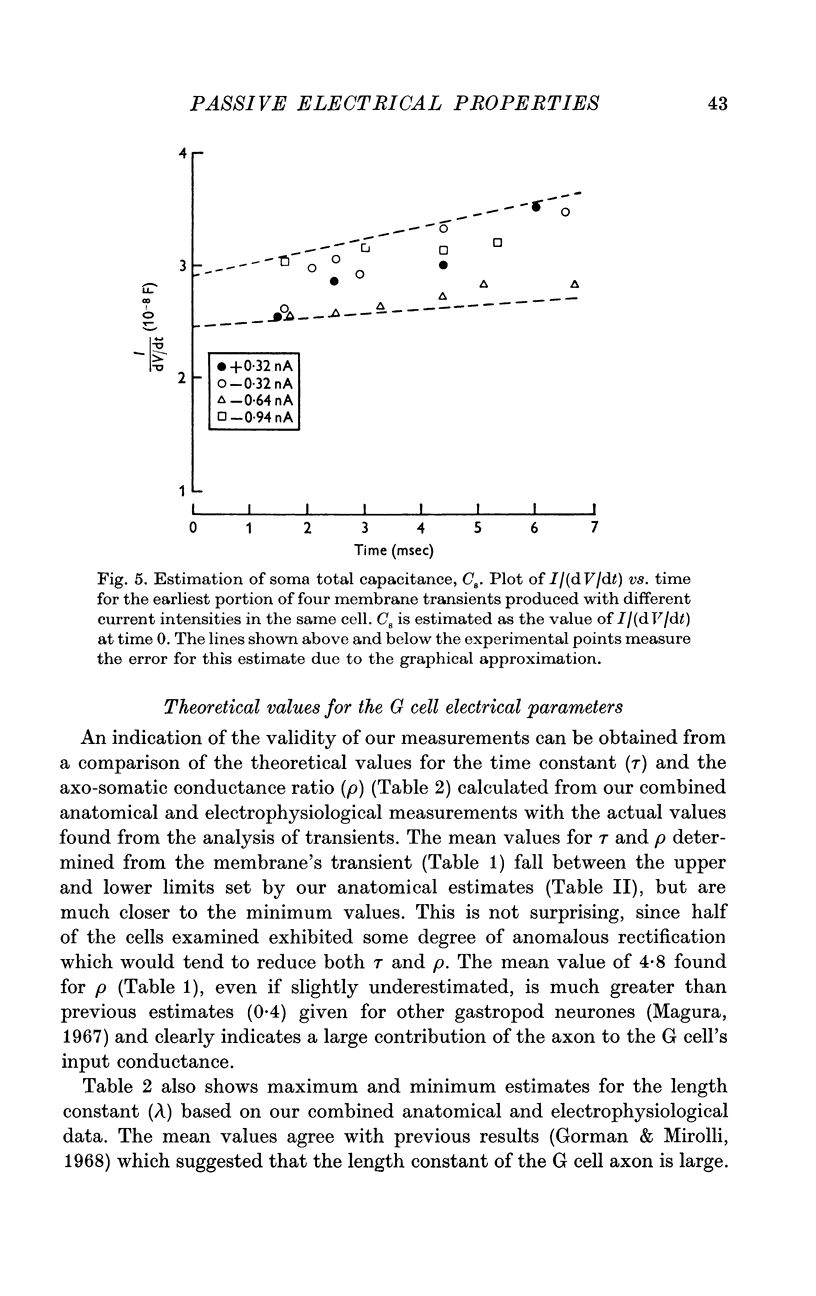
4. Estimates of specific membrane capacity, either from measurements of the initial portion of the membrane transient or from the ratio of the time constant to the specific membrane resistance are close to the value of 1 μF/cm2 expected for biological membranes.
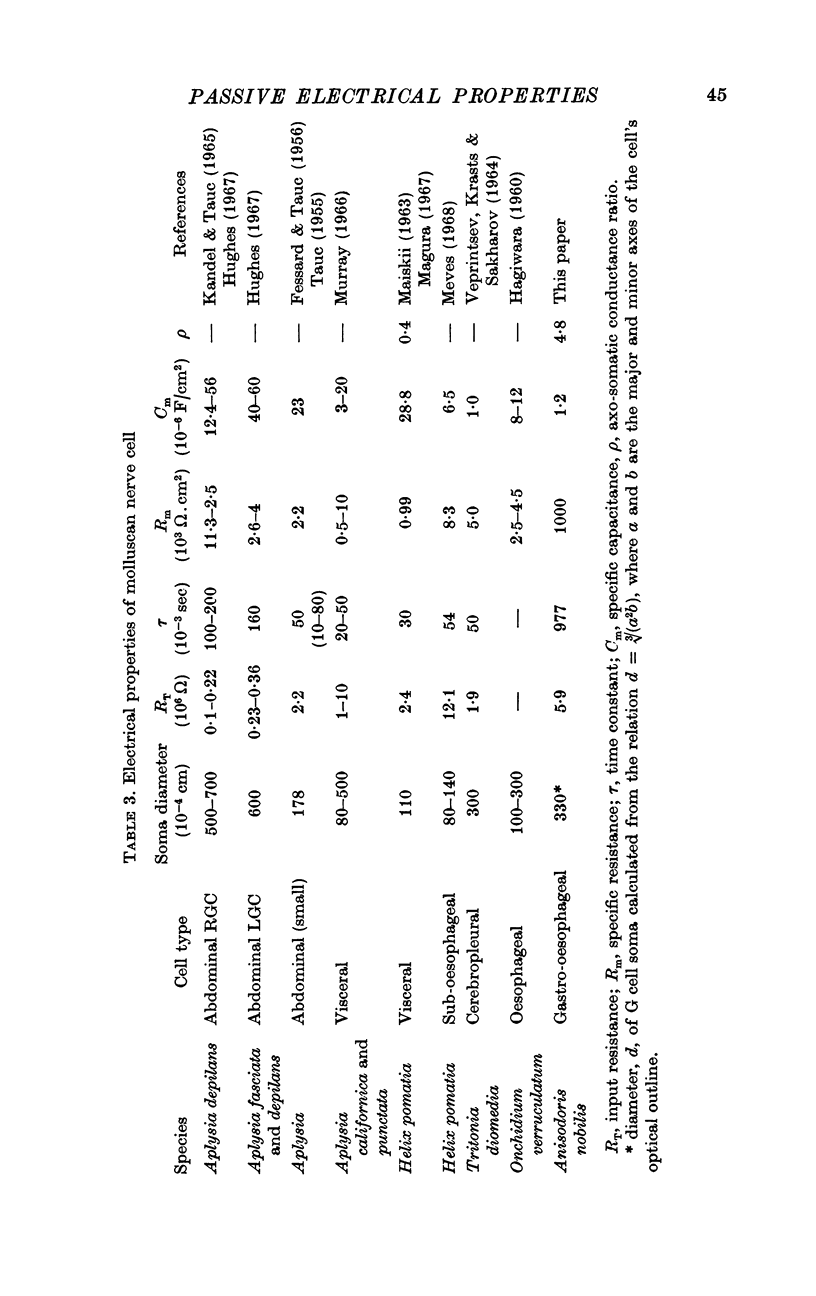
5. Thus, our measurements of specific capacitance, time constant, length constant and axo-somatic conductance ratio all indicate that the value found for the specific membrane resistance of the G cell, while unexpectedly large, is valid.
6. The magnitude of this value suggests that the conductance (permeability) of its membrane to ions is much smaller than that previously assumed for nerve membranes; this small conductance may be related to the larger surface-to-volume ratio of the G cell.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter D. O., Hovey M. M., Bak A. F. Intracellular conductance of Aplysia neurons and squid axon as determined by a new technique. Int J Neurosci. 1971 Jul;2(1):35–48. doi: 10.3109/00207457109146991. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E. A light and electron microscope study of the abdominal ganglion of Aplysia californica. J Neurophysiol. 1967 Nov;30(6):1263–1287. doi: 10.1152/jn.1967.30.6.1263. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C. Membrane time constants of cat motoneurons and time courses of synptic action. Exp Neurol. 1961 Jul;4:1–22. doi: 10.1016/0014-4886(61)90074-7. [DOI] [PubMed] [Google Scholar]
- FESSARD A., TAUC L. Capacité, résistance et variations actives d'impédance d'un soma neuronique. J Physiol (Paris) 1956 May-Jun;48(3):541–544. [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Mirolli M. Axonal localization of an excitatory post-synaptic potential in a molluscan neurone. J Exp Biol. 1970 Dec;53(3):727–736. doi: 10.1242/jeb.53.3.727. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Mirolli M. Electrotonic conduction in molluscan nerve cells. Experientia. 1968 Jul 15;24(7):693–694. doi: 10.1007/BF02138318. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L. The membrane resistance of a non-medullated nerve fibre. J Physiol. 1947 Jul 31;106(3):305–318. doi: 10.1113/jphysiol.1947.sp004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Mechanism of heterosynaptic facilitation in the giant cell of the abdominal ganglion of Aplysia depilans. J Physiol. 1965 Nov;181(1):28–47. doi: 10.1113/jphysiol.1965.sp007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Pollen D. A. Electrical constants of neurons in the motor cortex of the cat. J Neurophysiol. 1966 Mar;29(2):207–220. doi: 10.1152/jn.1966.29.2.207. [DOI] [PubMed] [Google Scholar]
- MAISKII V. A. ELEKTRICHESKIE KHARAKTERISTIKE POVERKHNOSTNO I MEMBRANY GIGANTSKIKH NERVNYKH KLETOK HELIX POMATIA. Fiziol Zh SSSR Im I M Sechenova. 1963 Dec;49:1468–1474. [PubMed] [Google Scholar]
- Magura I. S. O kolichestvennoi otsenke ionnykh tokov cherez membranu somy gigantskikh neironov legochnogo molliuska Planorbis corneus vo vremia generatsii potentsiala deistviia. Biofizika. 1967 May-Jun;12(3):456–461. [PubMed] [Google Scholar]
- Marmor M. F. The effects of temperature and ions on the current-voltage relation and electrical characteristics of a molluscan neurone. J Physiol. 1971 Nov;218(3):573–598. doi: 10.1113/jphysiol.1971.sp009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F. The independence of electrogenic sodium transport and membrane potential in a molluscan neurone. J Physiol. 1971 Nov;218(3):599–608. doi: 10.1113/jphysiol.1971.sp009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Mirolli M., Talbott S. R. The geometrical factors determining the electrotonic properties of a molluscan neurone. J Physiol. 1972 Dec;227(1):19–34. doi: 10.1113/jphysiol.1972.sp010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. W. The effect of temperature on the membrane properties of neurons in the visceral ganglion of Aplysia. Comp Biochem Physiol. 1966 Jun;18(2):291–303. doi: 10.1016/0010-406x(66)90188-5. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Brown A. M. Active transport of potassium and chloride in an identifiable molluscan neuron. Science. 1972 Mar 31;175(4029):1475–1477. doi: 10.1126/science.175.4029.1475. [DOI] [PubMed] [Google Scholar]
- TAUC L. Etude de l'activité élémentaire des cellules du ganglion abdominal de l'Aplysie. J Physiol (Paris) 1955;47(4):769–792. [PubMed] [Google Scholar]
- TAUC L. Site of origin and propagation in spike in the giant neuron of Aplysia. J Gen Physiol. 1962 Jul;45:1077–1097. doi: 10.1085/jgp.45.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEPRINTSEV B. N., KRASTS I. V., SAKHAROV D. A. NERVNYE KLETKI GOLOZHABERNOGO MOLLIUSKA TRITONIA DIOMEDIA BERGH. Biofizika. 1964;9:327–336. [PubMed] [Google Scholar]


