Abstract
1. Changes in choline concentration of the blood after injections or infusions of choline were studied in cats anaesthetized with chloralose.
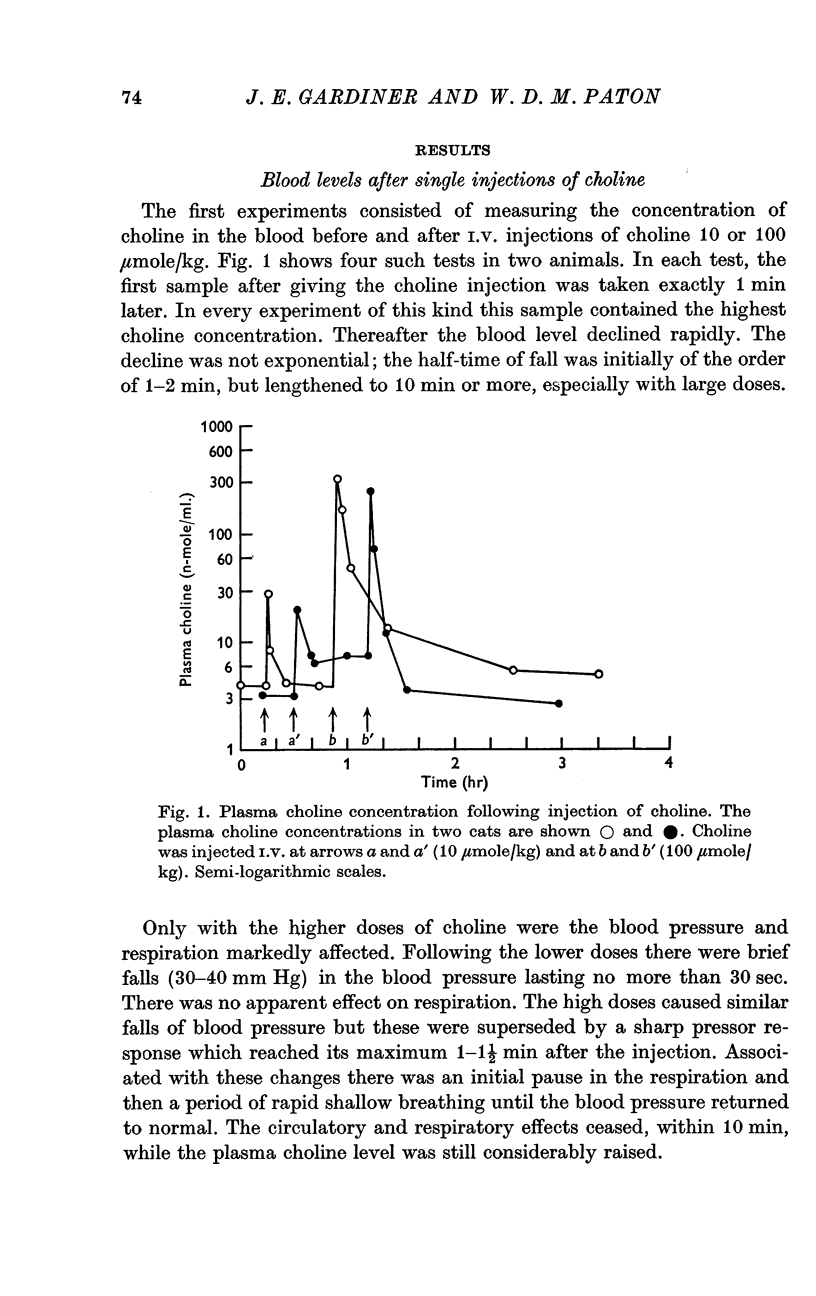
2. Single I.V. injections of choline 10-100 μmole/kg produced arterial plasma levels 1 min later corresponding to an apparent volume of initial distribution of 430 ml./kg. The concentration then declined rapidly (half-time, 1-2 min), with a later slower decline after large doses.
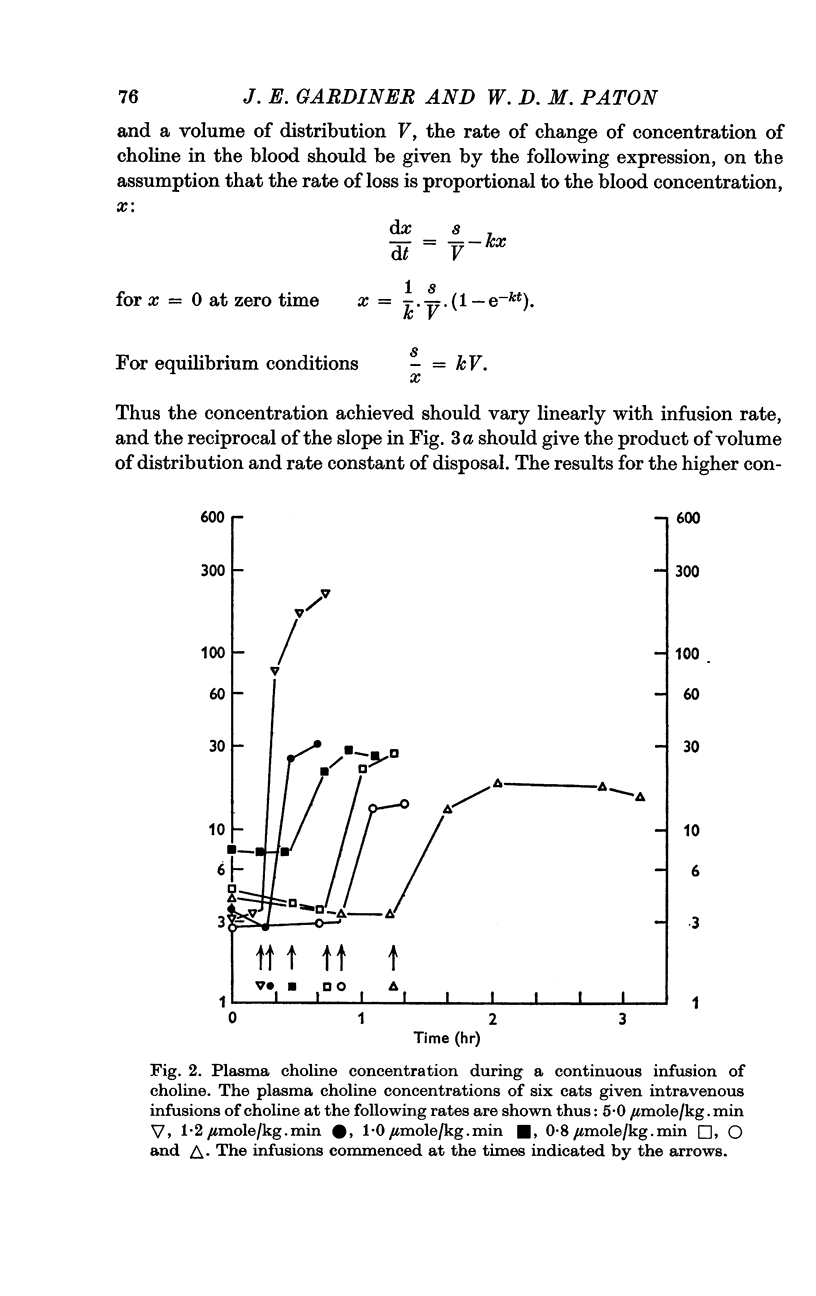
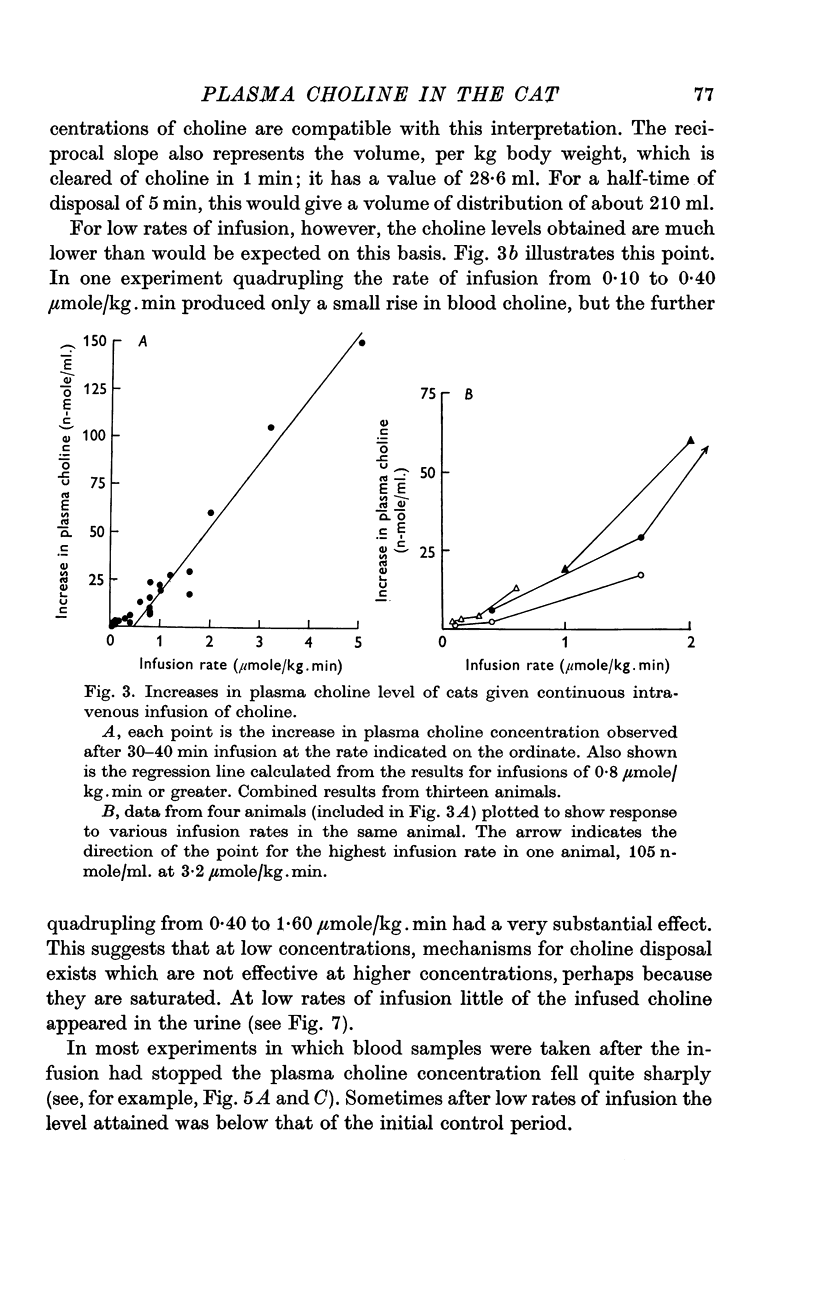
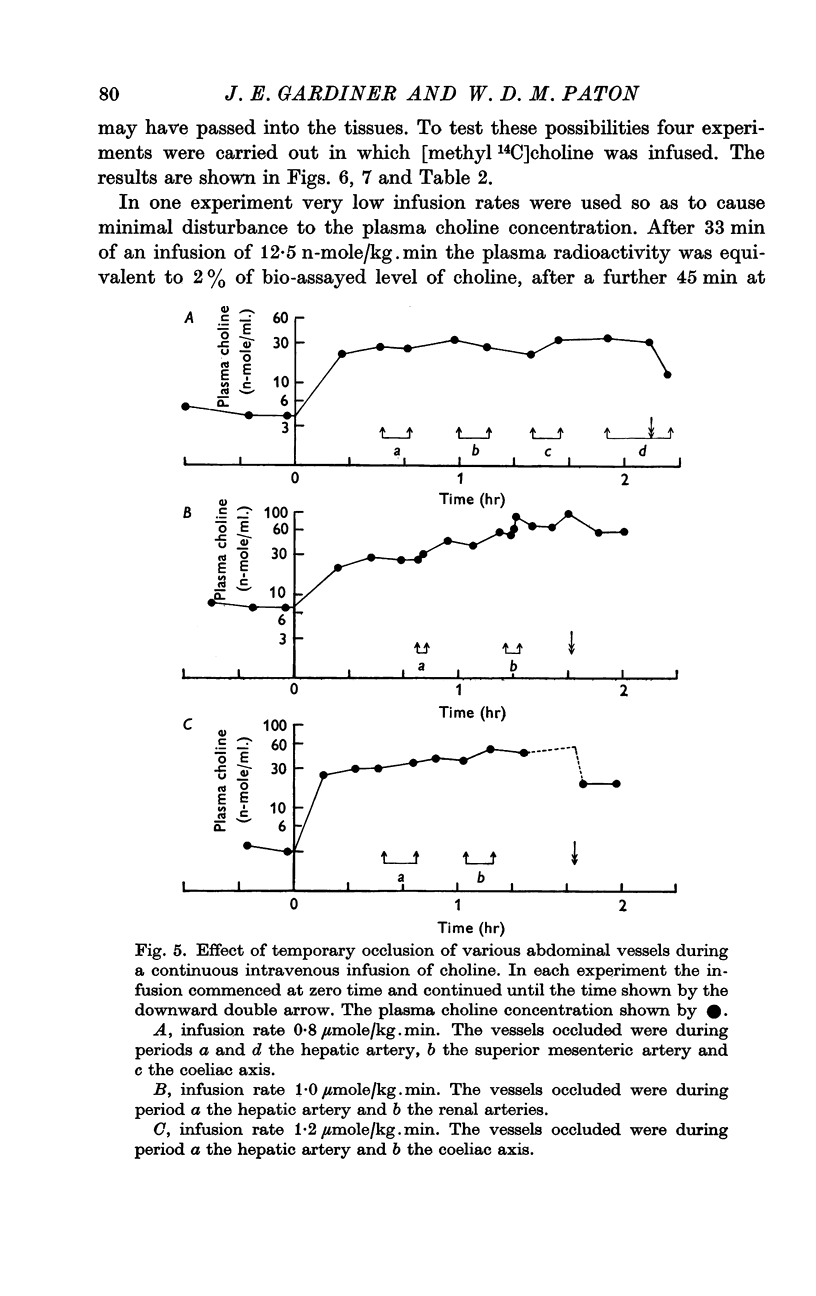
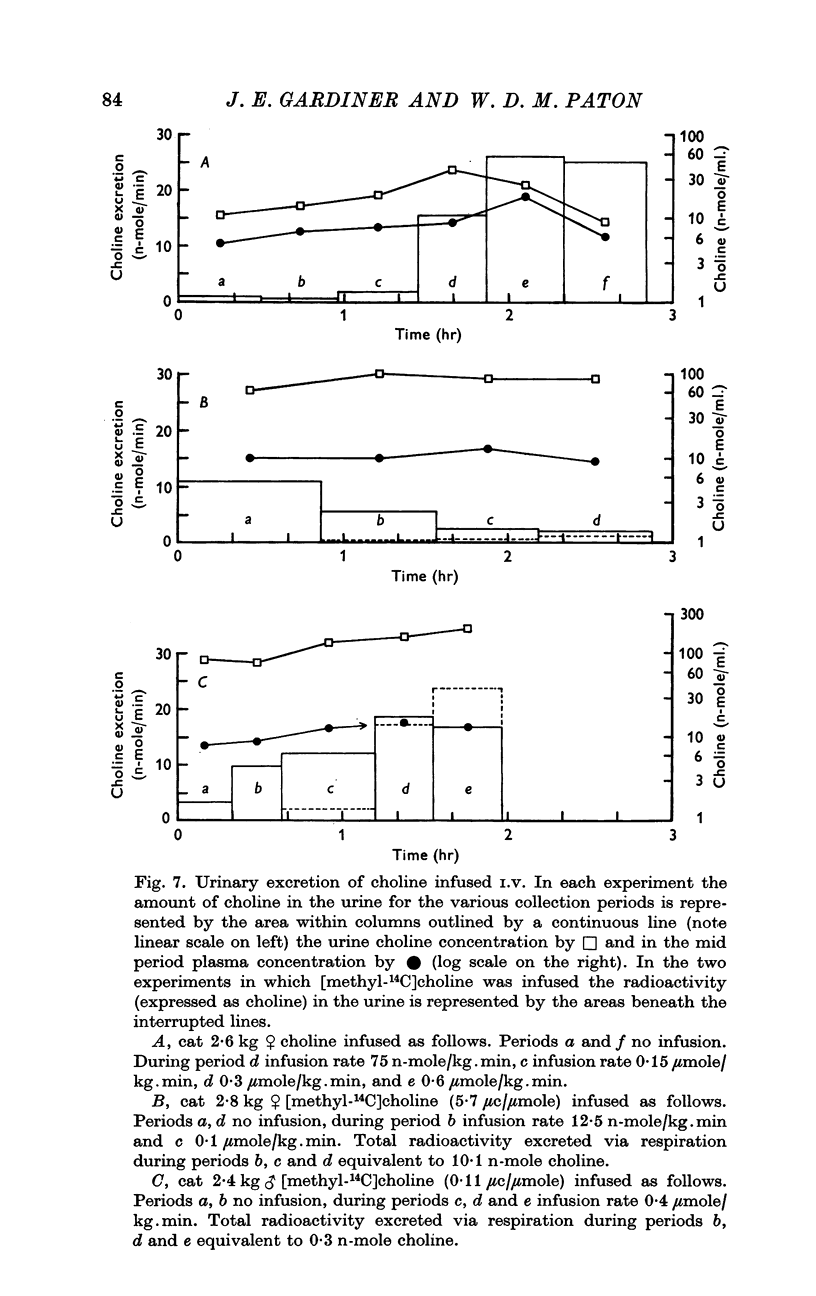
3. Infusions of choline at a rate of 0·8 μmole/kg.min or greater produced steady rises in plasma level, corresponding to a clearance of 28·6 ml. plasma/kg.min. The half time of rate of approach to steady state was 7 min or less. Infusions at rates of 0·40 μmole/kg.min or less produced much smaller or negligible rises, suggesting mechanisms for disposal which were saturated at higher concentration. At low rates, little infused choline appeared in urine. At the end of an infusion, the plasma choline level usually fell without delay.
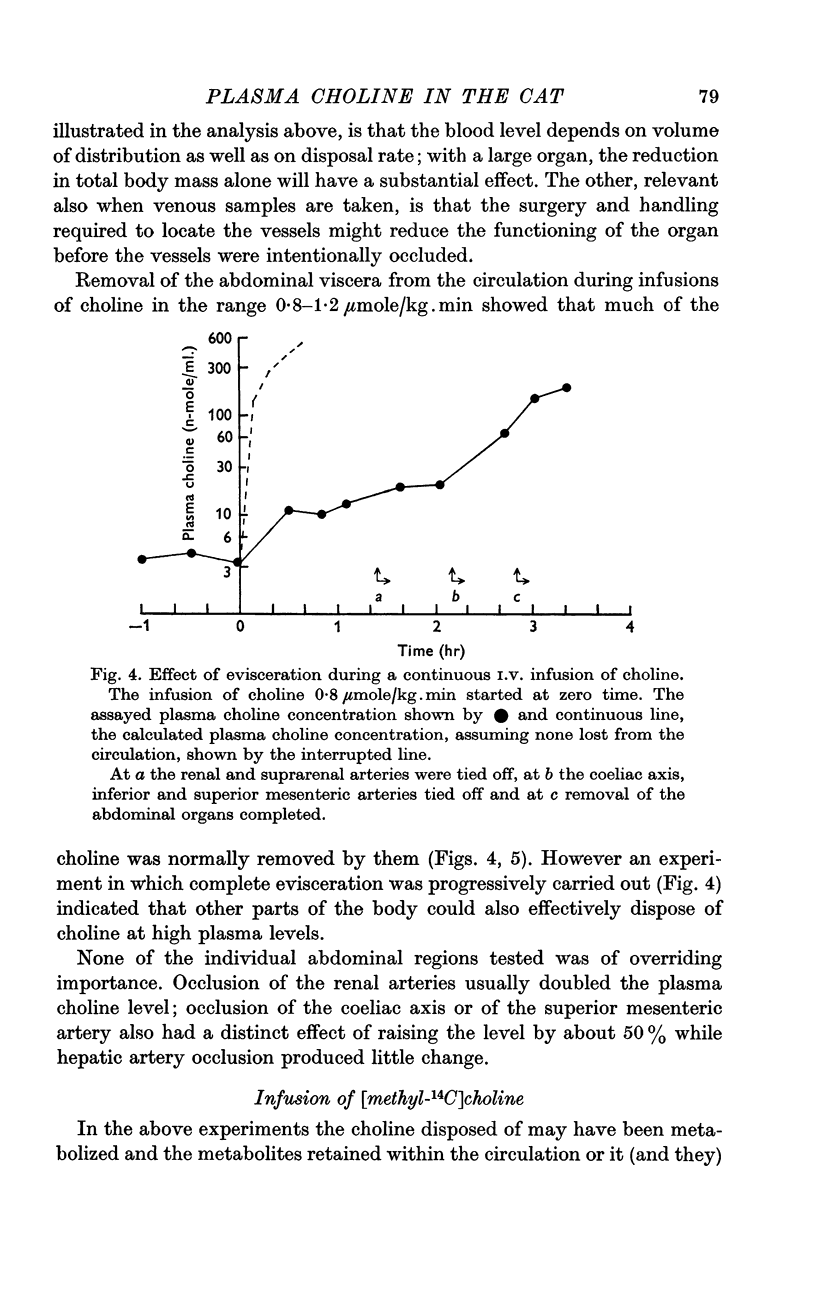
4. Portal blood contained about 50% of the arterial level, renal venous blood 15-70%, caval blood 30-60%, and amniotic fluid 2·5%. Occlusion of renal coeliac or mesenteric arteries raised plasma choline, but relatively rapid choline removal still occurred in the eviscerate animal.
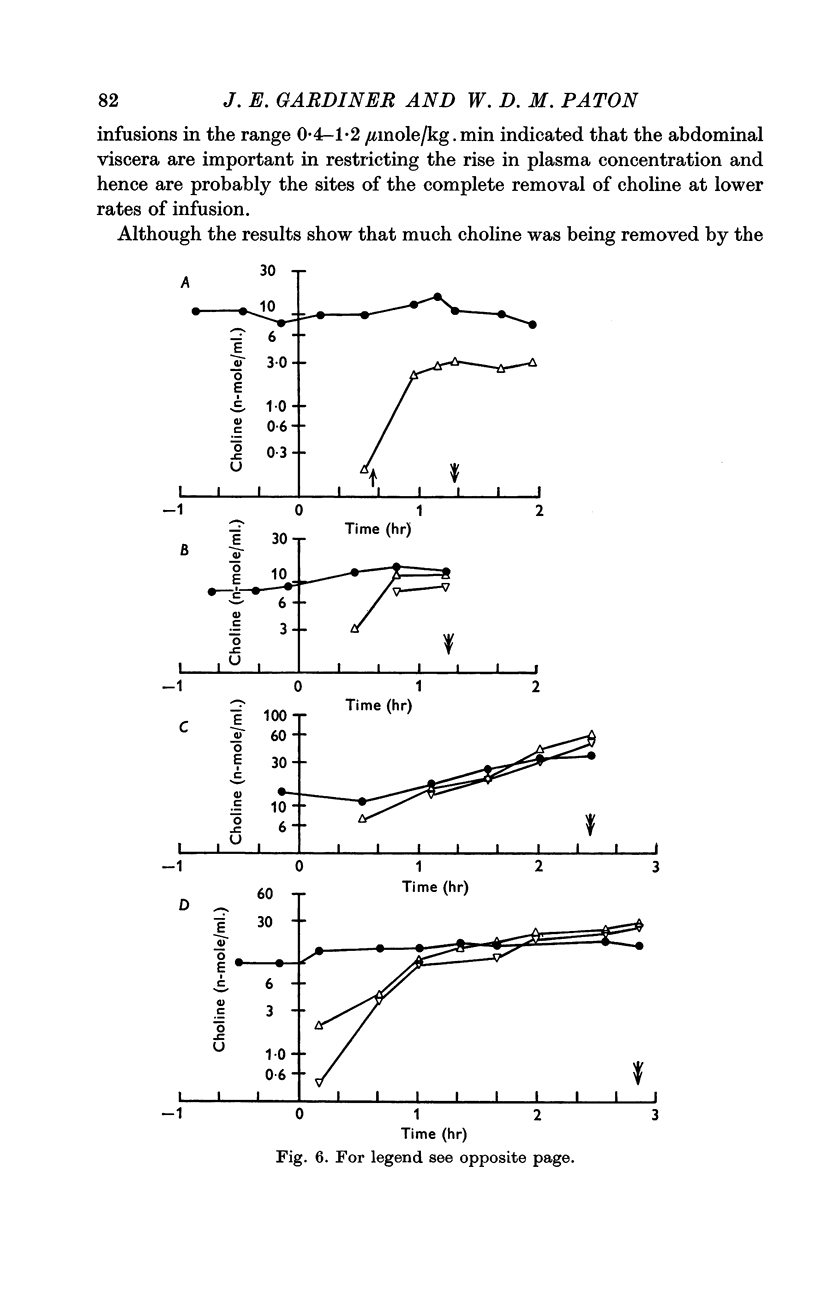
5. After infusions of [methyl-14C]choline, the level of radioactivity retained in the circulation amounted to only a few per cent of the total dose infused. At low rates of infusion (0·0125-0·1 μmole/kg.min) the radioactivity represented only a small fraction of bio-assayable choline; but at 0·40 μmole/kg.min it came to exceed the concentration of free choline, indicating metabolic conversion. Only traces of 14C were found in expired air, and only 1-1·5% of total infused radioactivity in the urine. After infusion of 150 μmole over 3 hr, high levels of radioactivity were found in liver, kidney, lung, brain and heart, but levels in muscle and spleen were comparable to that of blood.
6. It was concluded that choline is rapidly lost from the blood, that the abdominal viscera, liver, kidney and lung are important extraction sites, that some partial metabolism occurs, the metabolites also being rapidly lost from blood, and that it is probable that choline lost to the tissues becomes bound in some form.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH J. The level of free choline in plasma. J Physiol. 1952 Jun;117(2):234–240. doi: 10.1113/jphysiol.1952.sp004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH J. The role of the liver and the kidneys in the maintenance of the level of free choline in plasma. J Physiol. 1953 Apr 28;120(1-2):53–62. doi: 10.1113/jphysiol.1953.sp004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURA A., MONGAR J. L., SCHILD H. O. Improved automatic apparatus for pharmacological assays on isolated preparations. Br J Pharmacol Chemother. 1954 Mar;9(1):24–30. doi: 10.1111/j.1476-5381.1954.tb00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I. Choline metabolism in brain. The role of choline transport and the effects of phenobarbital. Arch Neurol. 1971 Apr;24(4):333–339. doi: 10.1001/archneur.1971.00480340065007. [DOI] [PubMed] [Google Scholar]
- GARDINER J. E. The inhibition of acetylcholine synthesis in brain by a hemicholinium. Biochem J. 1961 Nov;81:297–303. doi: 10.1042/bj0810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY J. A. B., PATON W. D. M. The circulation time in the cat, studied by a conductivity method. J Physiol. 1949 Dec 15;110(1-2):173–193. doi: 10.1113/jphysiol.1949.sp004430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner J. E., Domer F. R. Movement of choline between the blood and cerebrospinal fluid in the cat. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):482–501. [PubMed] [Google Scholar]
- Gardiner J. E., Sung L. H. A p-terphenyl hemicholinium compound. Br J Pharmacol. 1969 May;36(1):171P–172P. doi: 10.1111/j.1476-5381.1969.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACINTOSH F. C. Effect of HC-3 on acetylcholine turnover. Fed Proc. 1961 Jul;20:562–568. [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUELER F. W. A new group of respiratory paralyzants. I. The "hemicholiniums". J Pharmacol Exp Ther. 1955 Oct;115(2):127–143. [PubMed] [Google Scholar]
- SCHUELER F. W. The mechanism of action of the hemicholiniums. Int Rev Neurobiol. 1960;2:77–97. doi: 10.1016/s0074-7742(08)60120-8. [DOI] [PubMed] [Google Scholar]
- Spitzer H. L., Morrison K., Norman J. R. The incorporation of L-[Me-14C]methionine and [Me-3H]choline into lung phosphatides. Biochim Biophys Acta. 1968 May 1;152(3):552–558. doi: 10.1016/0005-2760(68)90095-7. [DOI] [PubMed] [Google Scholar]
- VANDER A. J. Renal excretion of choline in the dog. Am J Physiol. 1962 Feb;202:319–324. doi: 10.1152/ajplegacy.1962.202.2.319. [DOI] [PubMed] [Google Scholar]


