Abstract
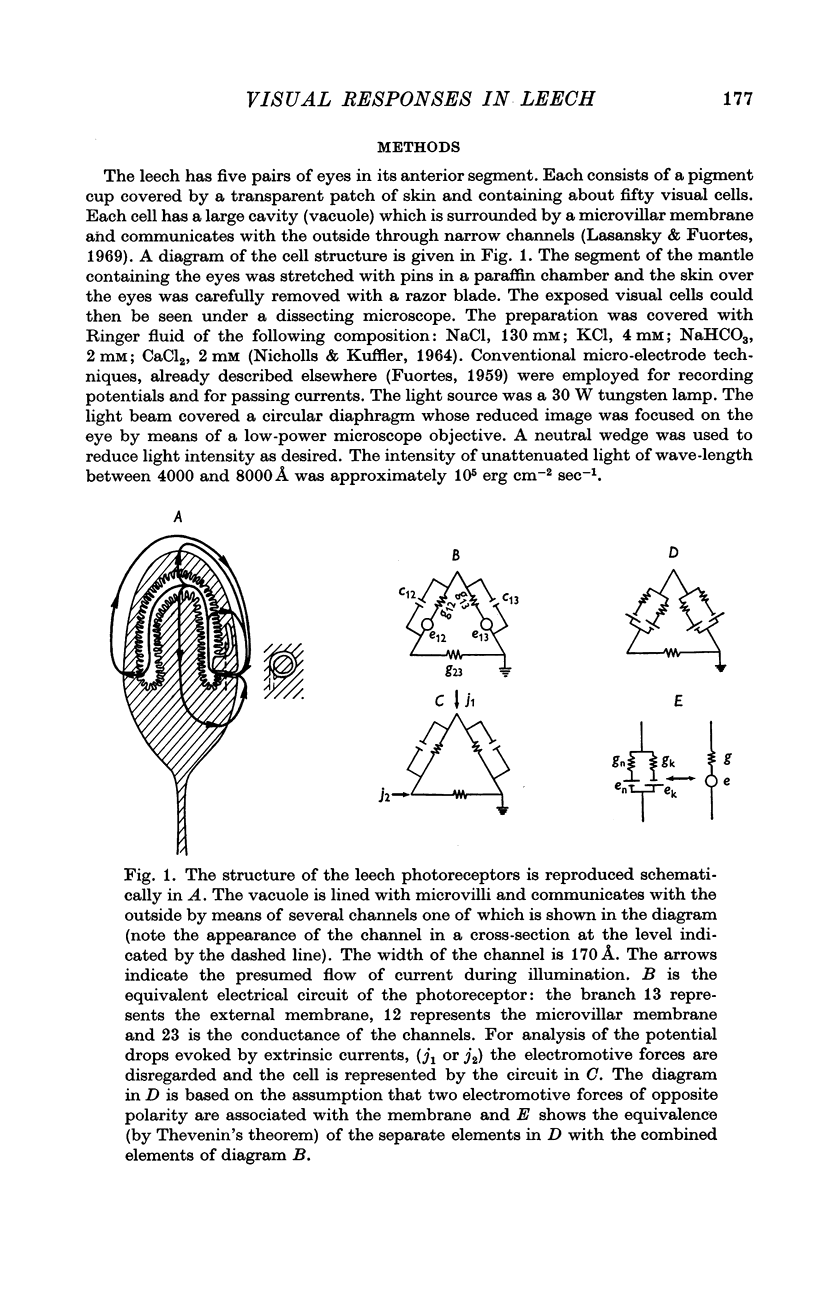
1. Potentials were recorded from the cytoplasm and from the vacuole of leech photoreceptors. Since the vacuole is lined with microvilli and is connected to the outside by narrow channels, the potential drops between vacuole and outside measure the current through the microvillar membrane.
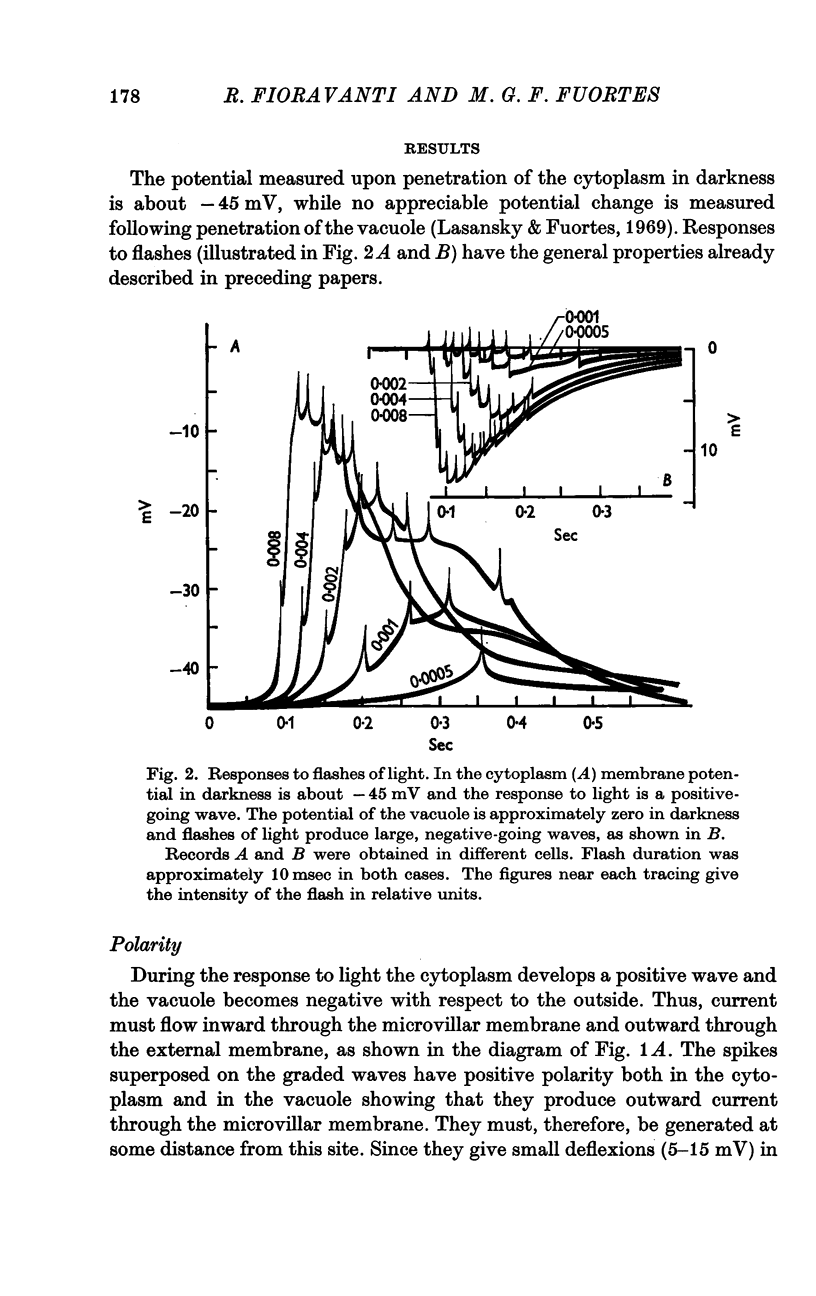
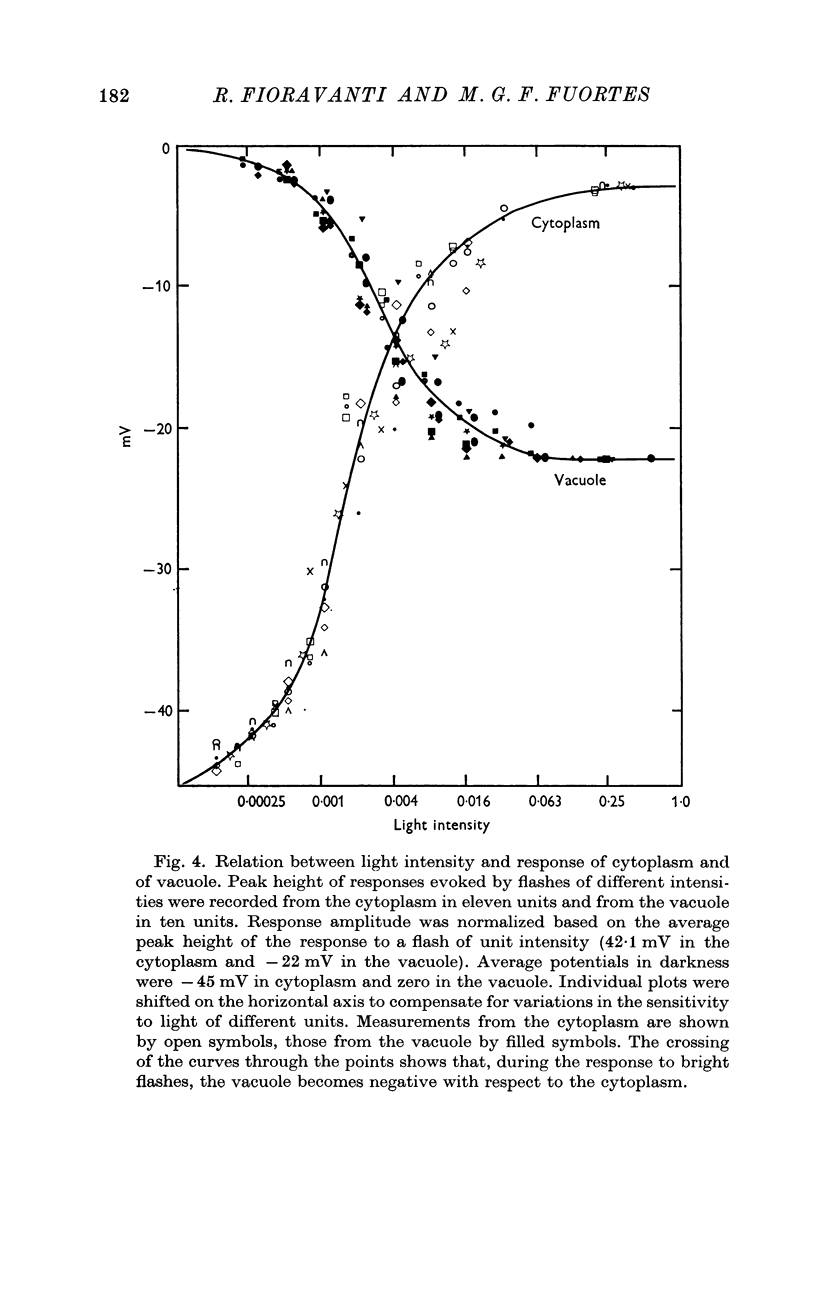
2. In darkness, the potential of the cytoplasm with respect to the outside is about — 45 mV while the potential of the vacuole is approximately zero.
3. Following illumination the negativity of the cytoplasm decreases and the vacuole becomes negative relative to the outside.
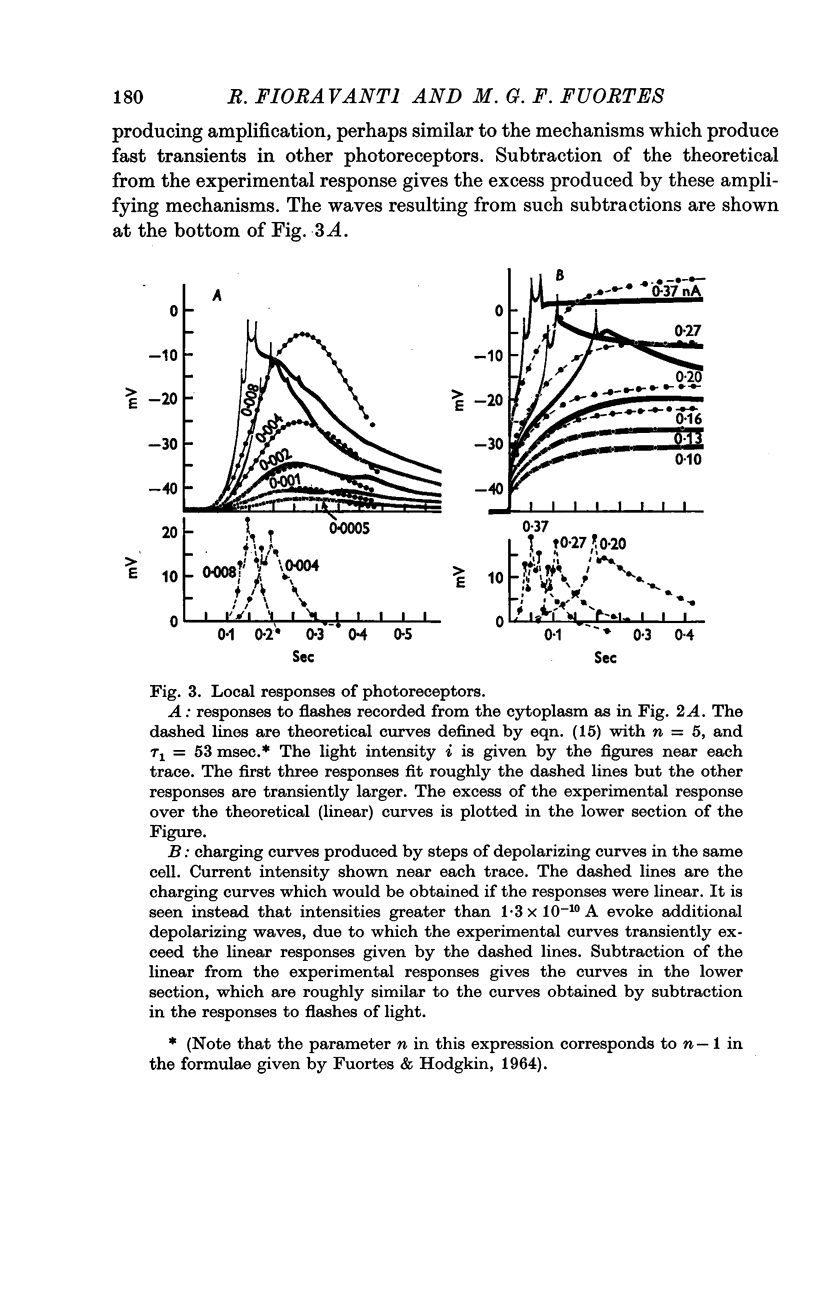
4. For dim intensities, the response to a flash of light may grow proportionately more than the intensity of the flash. This is probably due to development of a depolarizing local response.
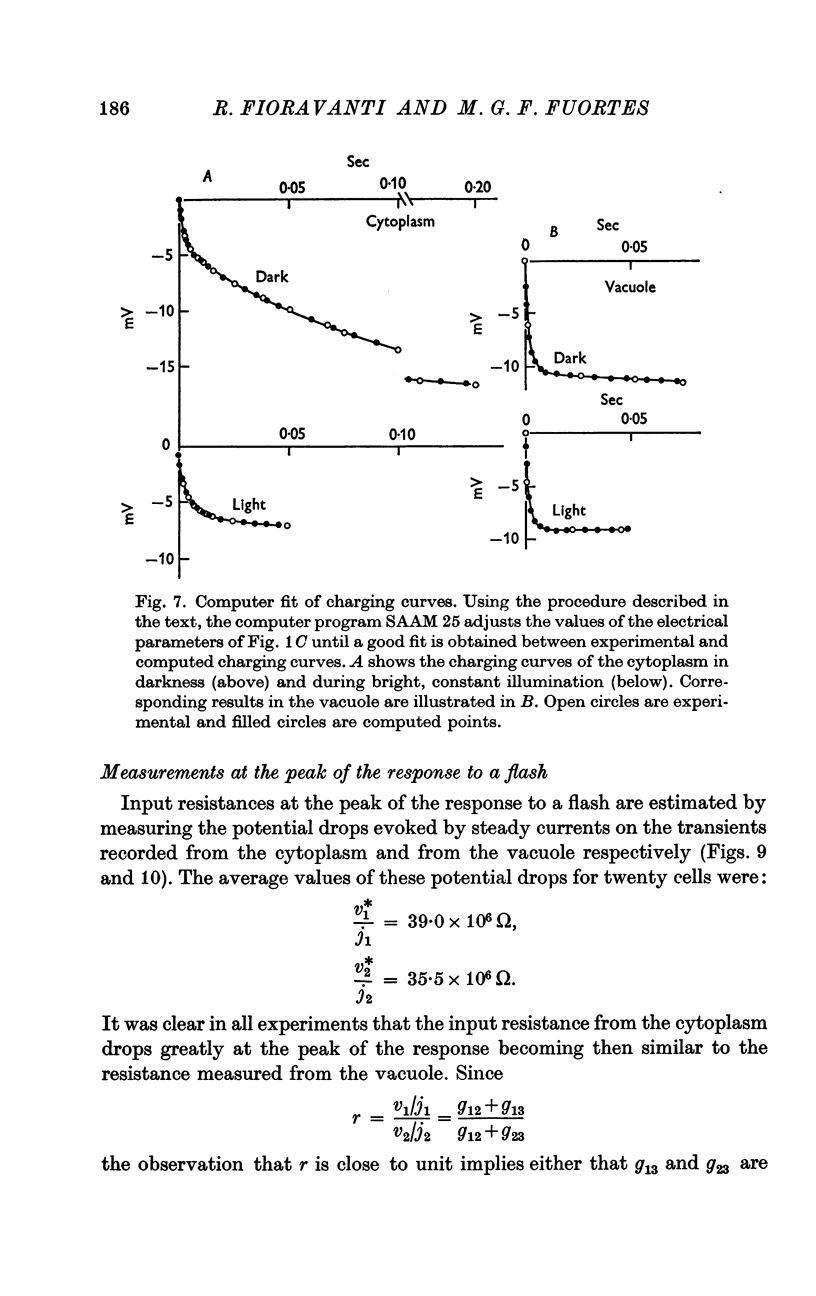
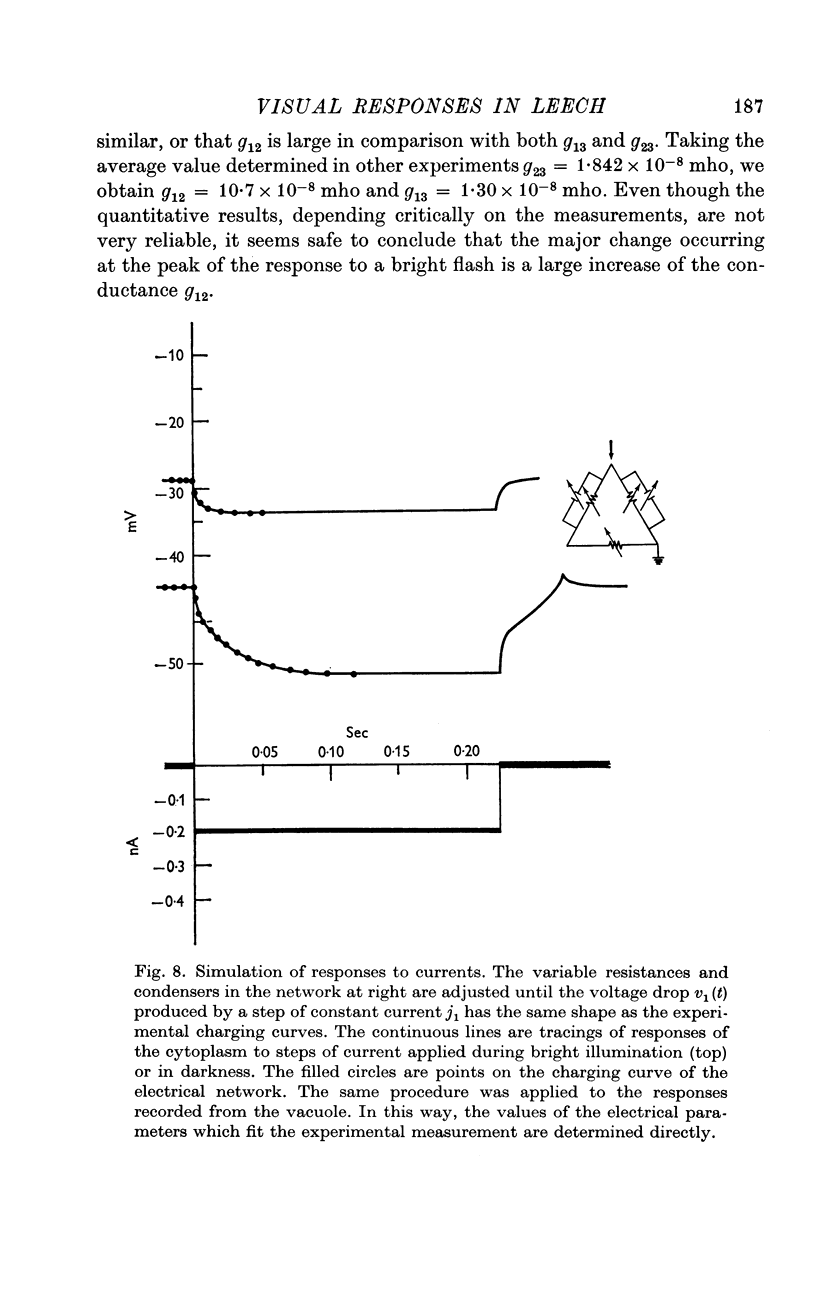
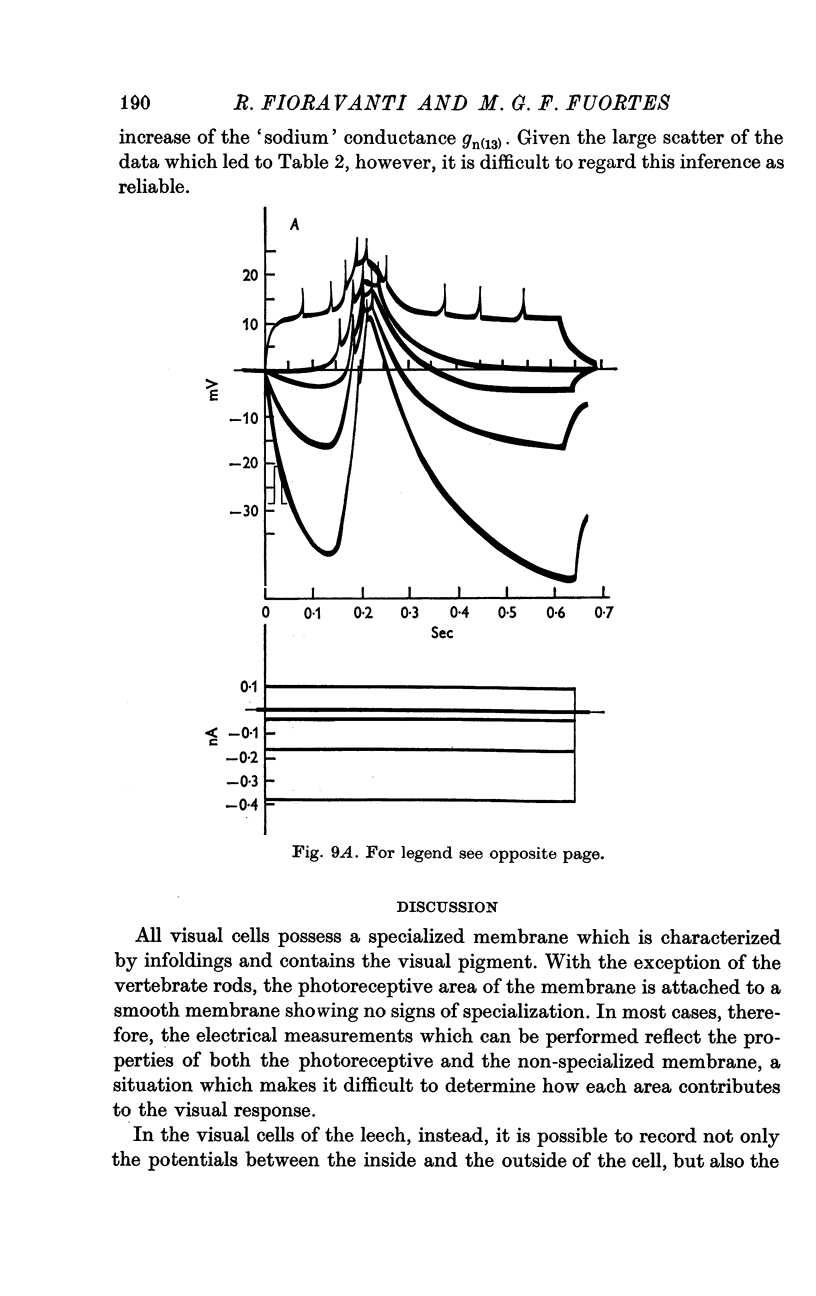
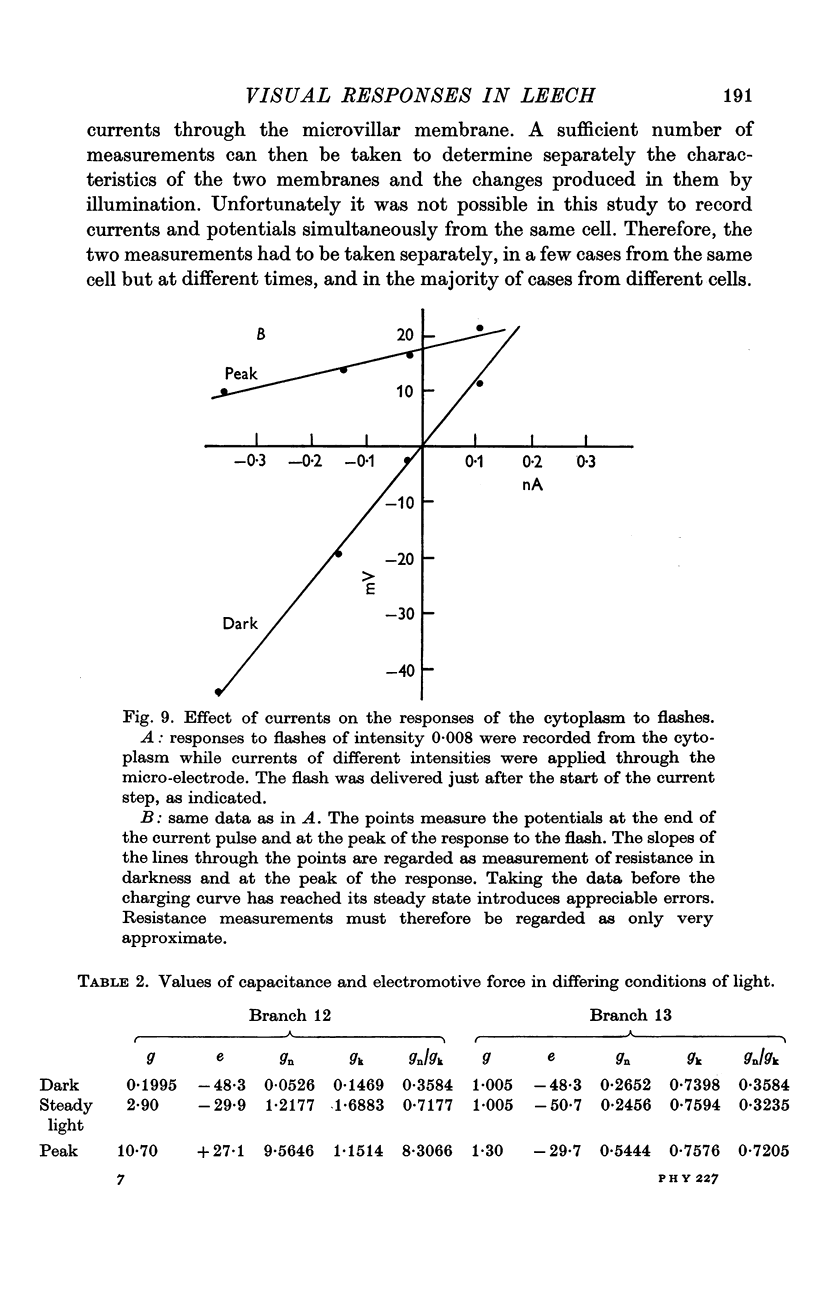
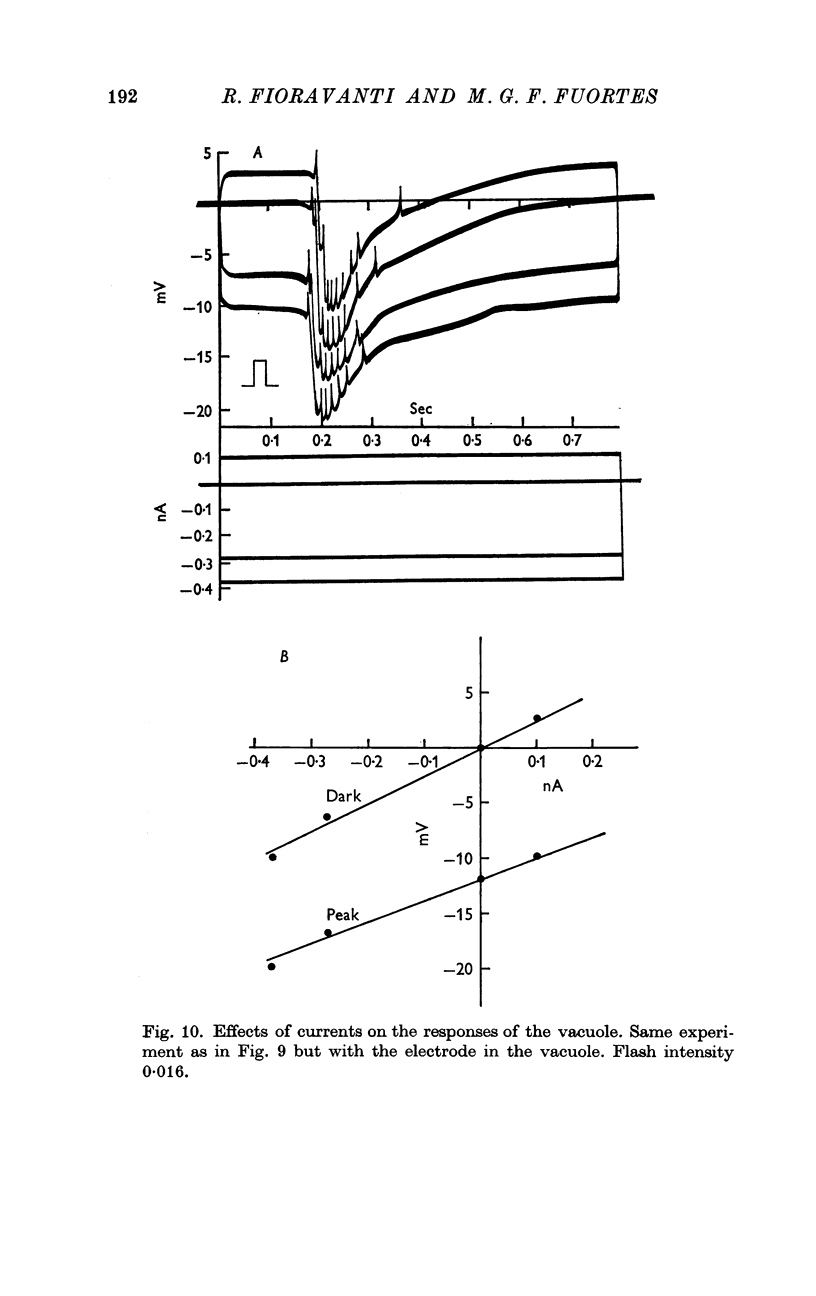
5. The resistance from the cytoplasm to the outside was about 150 MΩ in darkness and decreased to approximately 40 MΩ at the peak of the response to a bright flash (on average). Corresponding measurements from the vacuole gave 50 MΩ in darkness and 35 MΩ at the peak of the response.
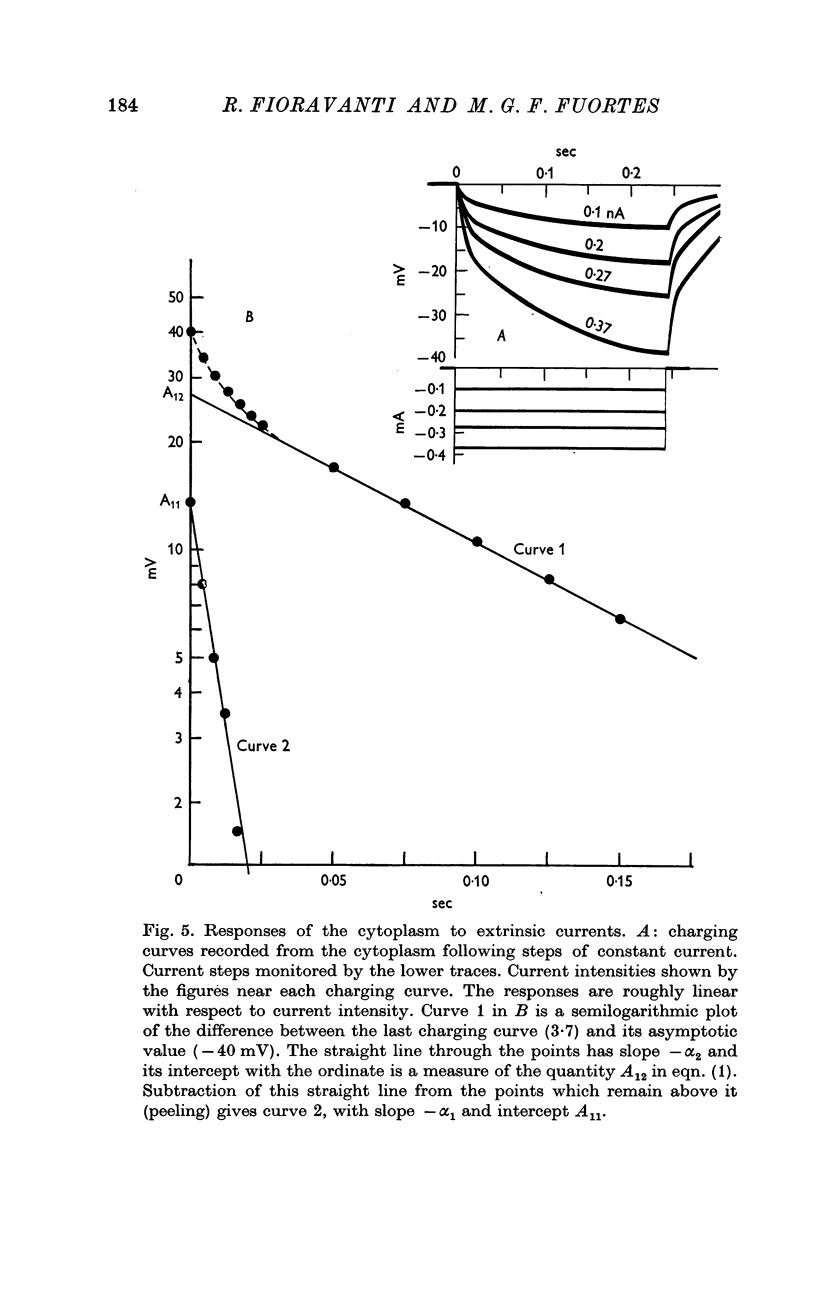
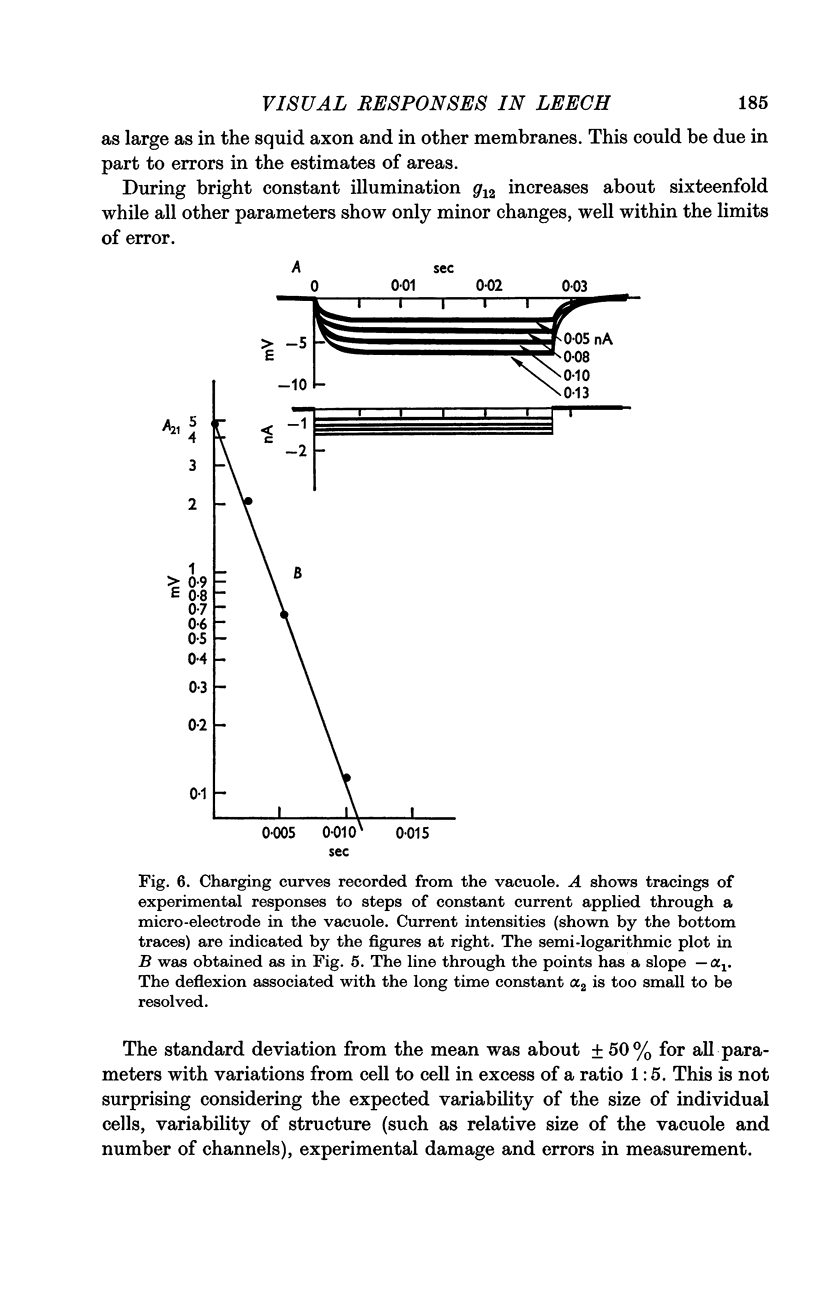
6. Charging curves produced by steps of constant currents applied to the cytoplasm or to the vacuole include two time constants (about 5 and 50 msec on average). The longer time constant decreases greatly with bright illumination.
7. The results are consistent with the interpretation that the response to light is brought about by an increase of conductance of the microvillar membrane.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann F. Slow and spike potentials recorded from retinula cells of the honeybee drone in response to light. J Gen Physiol. 1968 Dec;52(6):855–875. doi: 10.1085/jgp.52.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., FRANK K., BECKER M. C. Steps in the production of motoneuron spikes. J Gen Physiol. 1957 May 20;40(5):735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G. Initiation of impulses in visual cells of Limulus. J Physiol. 1959 Oct;148:14–28. doi: 10.1113/jphysiol.1959.sp006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Fuortes M. G. The site of origin of electrical responses in visual cells of the leech, Hirudo medicinalis. J Cell Biol. 1969 Jul;42(1):241–252. doi: 10.1083/jcb.42.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J. G., KUFFLER S. W. EXTRACELLULAR SPACE AS A PATHWAY FOR EXCHANGE BETWEEN BLOOD AND NEURONS IN THE CENTRAL NERVOUS SYSTEM OF THE LEECH: IONIC COMPOSITION OF GLIAL CELLS AND NEURONS. J Neurophysiol. 1964 Jul;27:645–671. doi: 10.1152/jn.1964.27.4.645. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. A theoretical treatment of Fuortes's observations upon eccentric cell activity in Limulus. J Physiol. 1959 Oct;148:29–38. doi: 10.1113/jphysiol.1959.sp006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA T. Peripheral mechanism of nervous activity in lateral eye of horseshoe crab. J Neurophysiol. 1957 May;20(3):245–254. doi: 10.1152/jn.1957.20.3.245. [DOI] [PubMed] [Google Scholar]


