Abstract
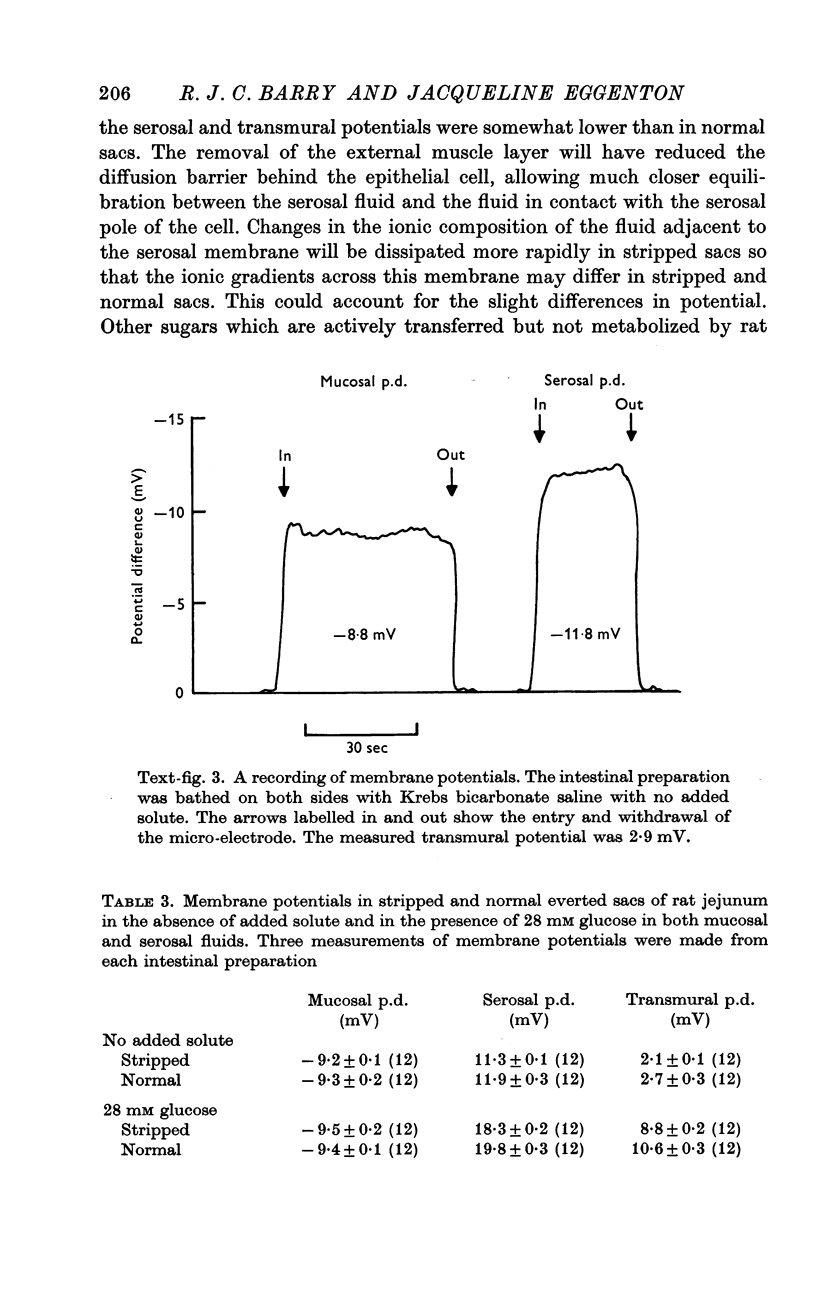
1. Stripped sacs of rat jejunum in which the outer muscle layers had been removed were found to maintain substantial transport and electrical activities.
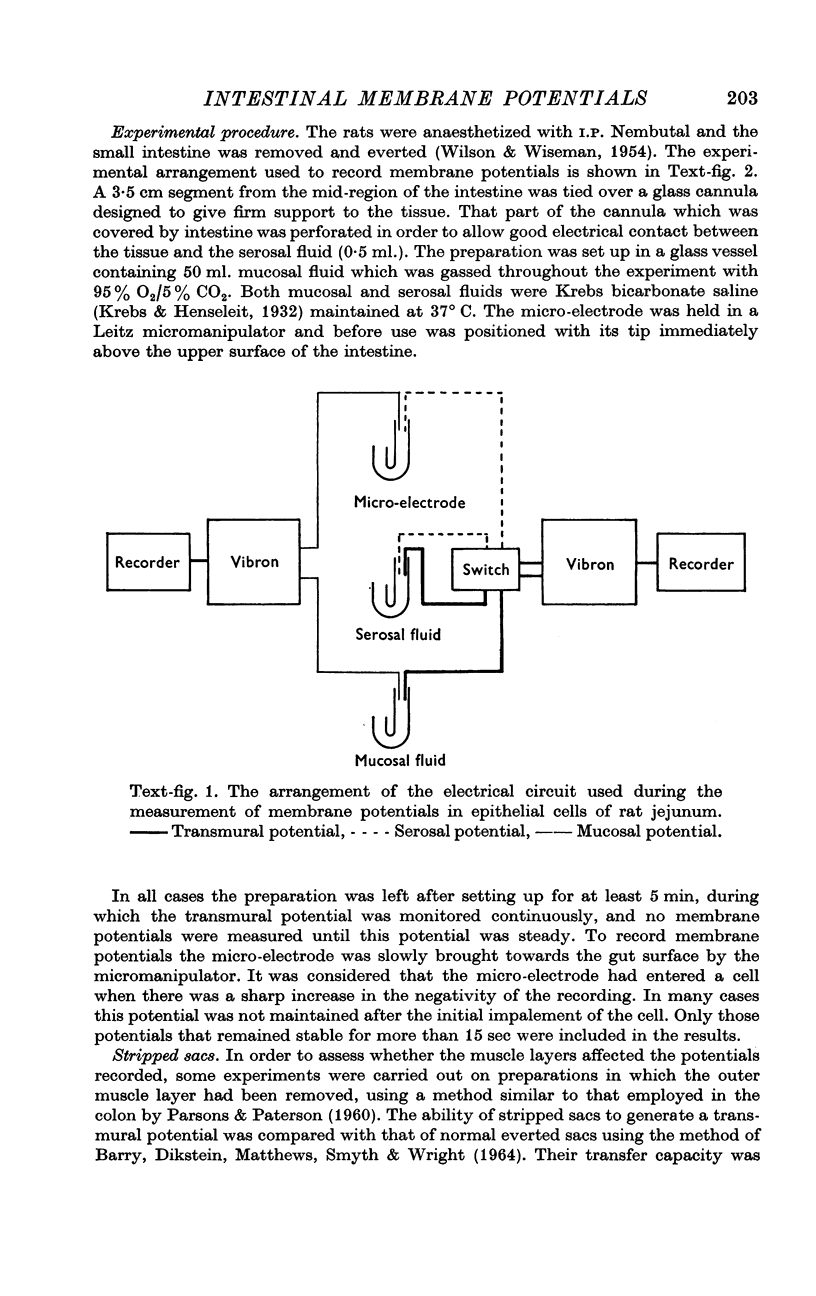
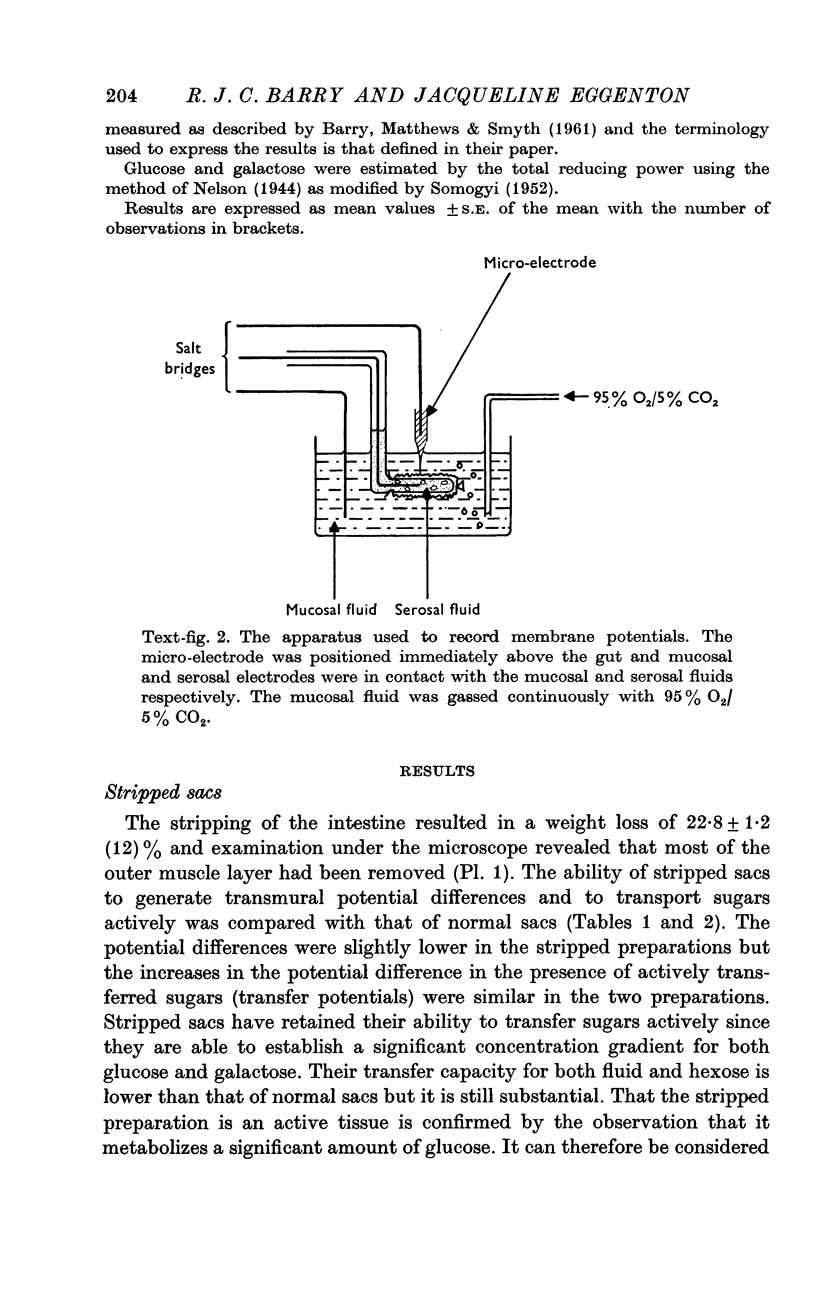
2. Mucosal and serosal membrane potentials of epithelial cells of normal and stripped everted sacs of rat jejunum were recorded in vitro together with the transmural potential difference.
3. The cell interior was negative relative to both serosal and mucosal fluids, the transmural potential being the sum of the two membrane potentials.
4. Changes in the transmural potentials in the presence of actively transferred hexoses and amino acids were entirely due to variations in the serosal potential, the mucosal potential being unchanged.
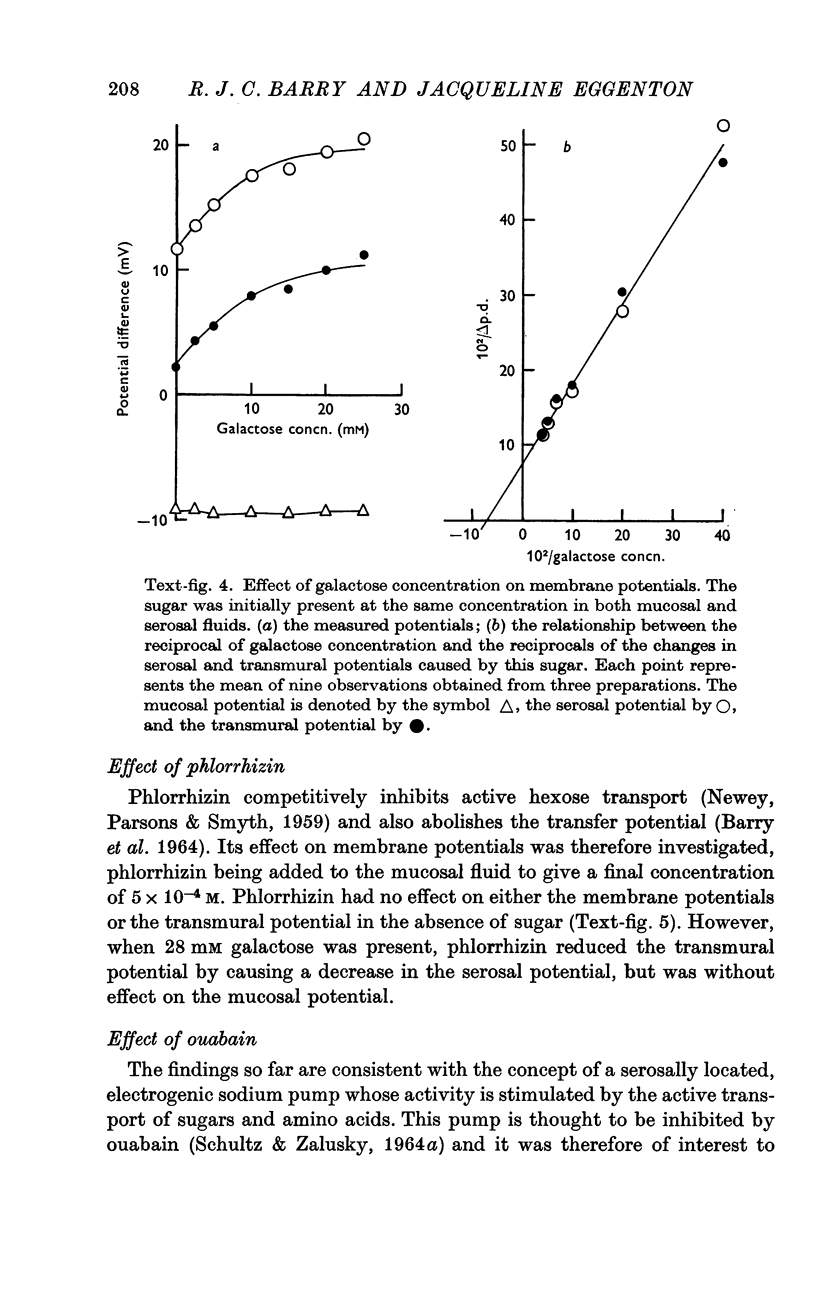
5. Serosal and transmural potential increases on the addition of galactose were consistent with Michaelis—Menten kinetics, giving apparent Km values of 14·9 and 14·1 mM respectively.
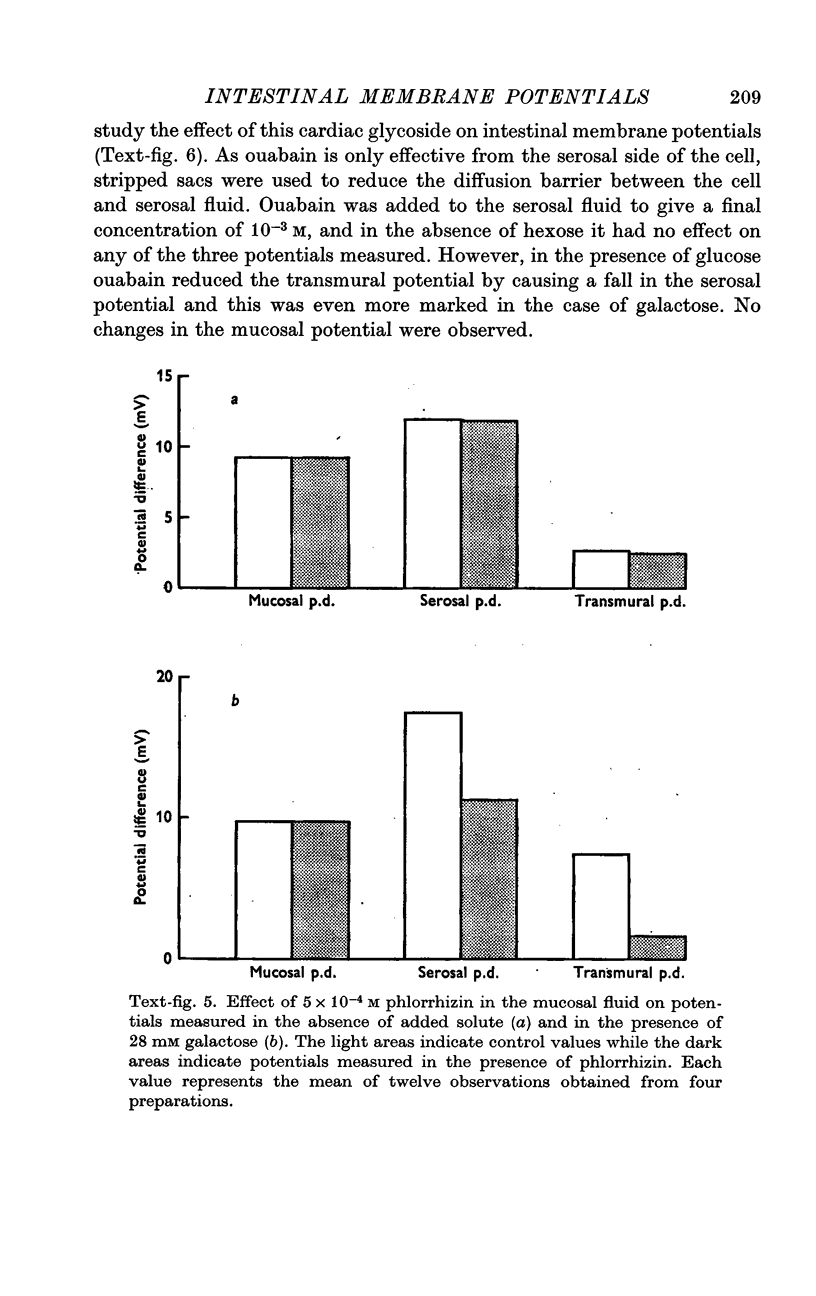
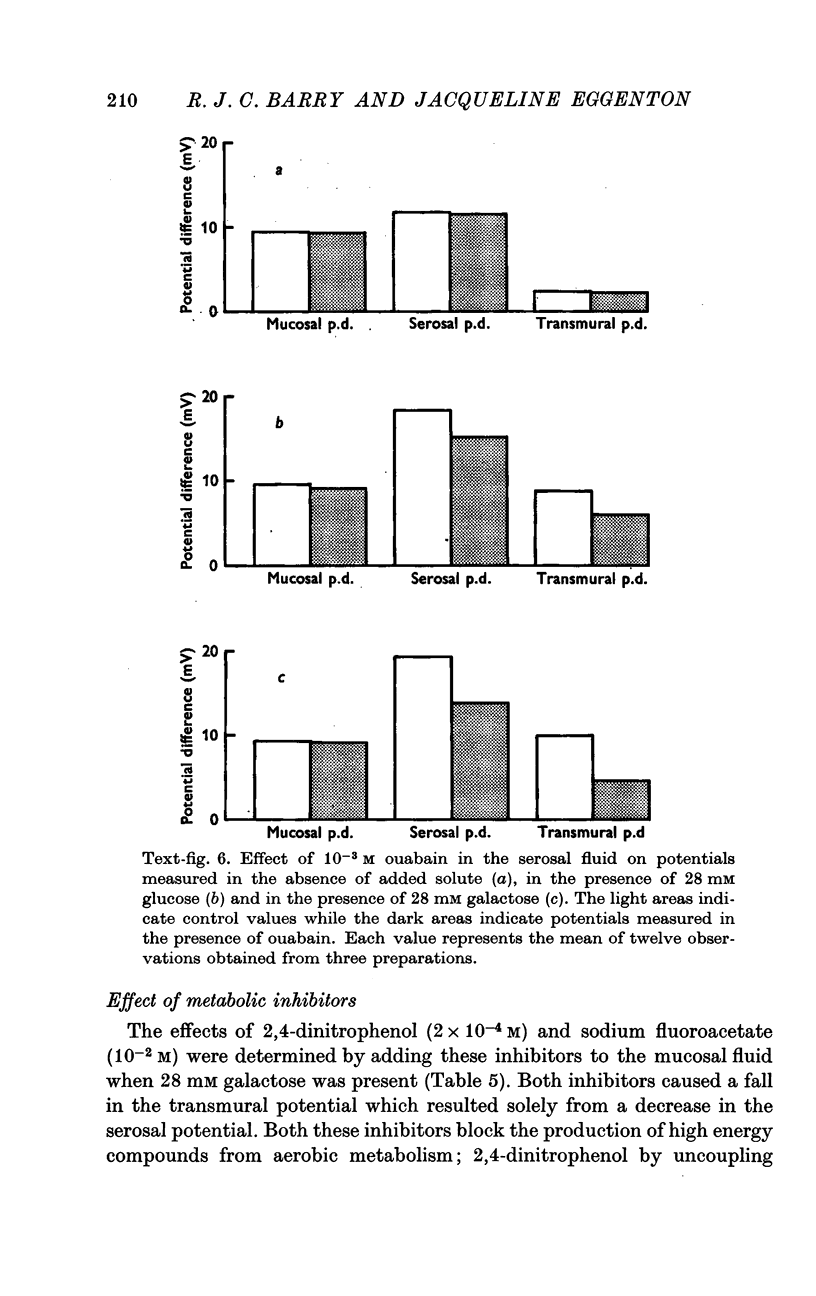
6. Phlorrhizin, ouabain, 2,4-dinitrophenol and sodium fluoroacetate inhibited serosal potential changes in the presence of galactose.
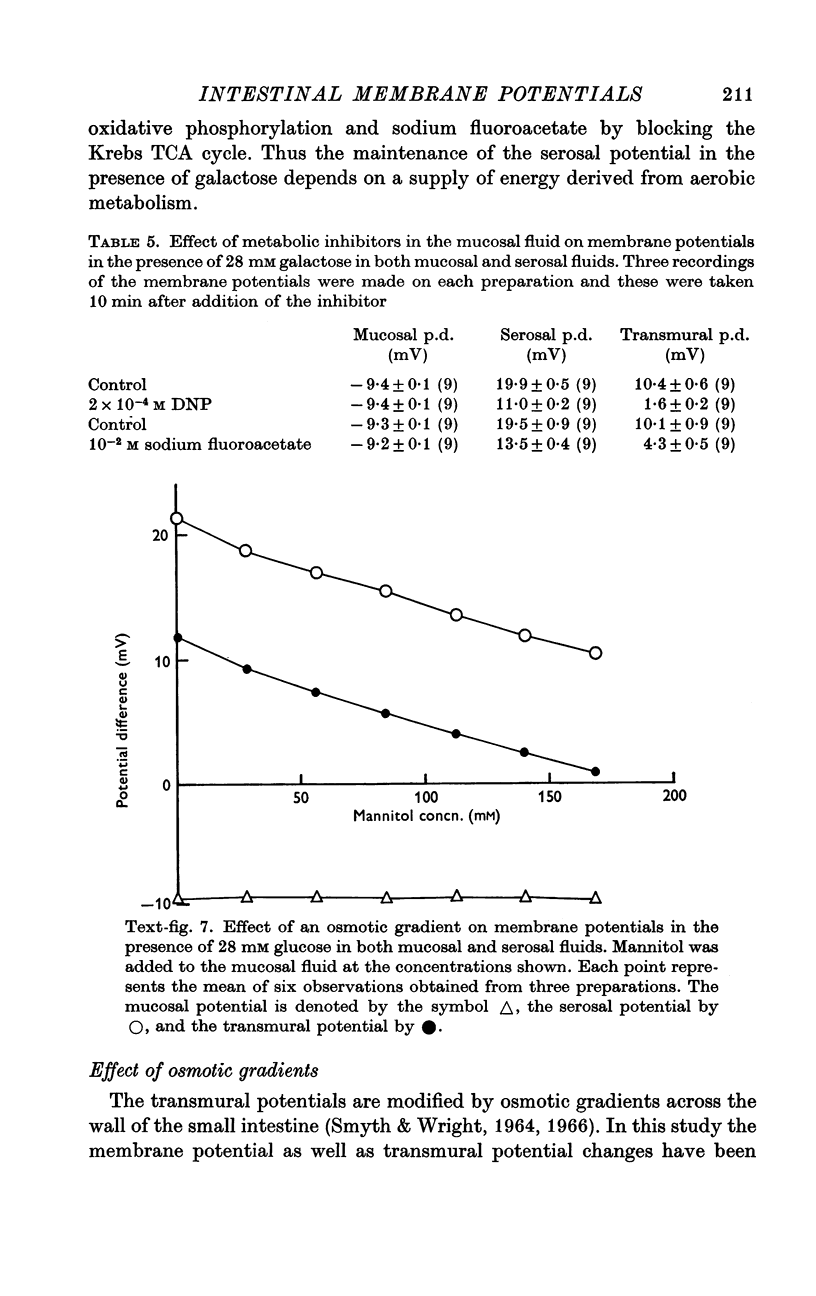
7. Osmotic potentials resulting from transmural osmotic gradients originated from the serosal layers of the tissue.
8. The results are consistent with the concept of a serosally located, electrogenic sodium pump which is stimulated by actively transferred hexoses and amino acids. The sodium-dependent entry mechanism at the mucosal membrane is non-electrogenic.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY B. A., MATTHEWS J., SMYTH D. H. Transfer of glucose and fluid by different parts of the small intestine of the rat. J Physiol. 1961 Jul;157:279–288. doi: 10.1113/jphysiol.1961.sp006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosacková J., Crane R. K. Studies on the mechanism of intestinal absorption of sugars. 8. Cation inhibition of active sugar transport and 22Na influx into hamster small intestine, in vitro. Biochim Biophys Acta. 1965 Jul 22;102(2):423–435. doi: 10.1016/0926-6585(65)90132-9. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J., Nielsen O. E. Transmembrane electrical potential differences and ionic composition of mucosal cells of rat colon. Acta Physiol Scand. 1968 Mar;72(3):338–349. doi: 10.1111/j.1748-1716.1968.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Gilles-Baillien M., Schoffeniels E. Site of action of L-alanine and D-glucose on the potential difference across the intestine. Arch Int Physiol Biochim. 1965 Mar;73(2):355–357. doi: 10.3109/13813456509084257. [DOI] [PubMed] [Google Scholar]
- Janáek K., Morel F., Bourguet J. Etude expérimentale des potentiels électriques et des activités ioniques dans les cellules épithéliales de la vessie de grenouille. J Physiol (Paris) 1968 Jan-Feb;60(1):51–66. [PubMed] [Google Scholar]
- Lyon I., Crane R. K. Studies on transmural potentials in vitro in relation to intestinal absorption. I. Apparent Michaelis constants for Na+dependent sugar transport. Biochim Biophys Acta. 1966 Feb 7;112(2):278–291. doi: 10.1016/0926-6585(66)90327-x. [DOI] [PubMed] [Google Scholar]
- Lyon I., Sheerin H. E. Studies on transmural potentials in vitro in relation to intestinal absorption. VI. The effect of sugars on electrical potential profiles in jejunum and ileum. Biochim Biophys Acta. 1971 Oct 12;249(1):1–14. doi: 10.1016/0005-2736(71)90078-2. [DOI] [PubMed] [Google Scholar]
- NEWEY H., PARSONS B. J., SMYTH D. H. The site of action of phlorrhizin in inhibiting intestinal absorption of glucose. J Physiol. 1959 Oct;148:83–92. doi: 10.1113/jphysiol.1959.sp006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS D. S., PATERSON G. R. Movements of fluid and glucose in an everted sac preparation of rat colonic mucosa. Biochim Biophys Acta. 1960 Jun 17;41:173–175. doi: 10.1016/0006-3002(60)90393-0. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. INTERACTIONS BETWEEN ACTIVE SODIUM TRANSPORT AND ACTIVE AMINO-ACID TRANSPORT IN ISOLATED RABBIT ILEUM. Nature. 1965 Jan 16;205:292–294. doi: 10.1038/205292a0. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Smyth D. H., Wright E. M. Streaming potentials in the rat small intestine. J Physiol. 1966 Feb;182(3):591–602. doi: 10.1113/jphysiol.1966.sp007839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., POLLEY E. H., ORREGO F. Action potentials from individual elements in cat geniculate and striate cortex. J Neurophysiol. 1954 Sep;17(5):454–474. doi: 10.1152/jn.1954.17.5.454. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDHAGER E. E., GIEBISCH G. ELECTROPHYSIOLOGY OF THE NEPHRON. Physiol Rev. 1965 Apr;45:214–244. doi: 10.1152/physrev.1965.45.2.214. [DOI] [PubMed] [Google Scholar]
- White J. F., Armstrong W. M. Effect of transported solutes on membrane potentials in bullfrog small intestine. Am J Physiol. 1971 Jul;221(1):194–201. doi: 10.1152/ajplegacy.1971.221.1.194. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Patterns of non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):227–271. doi: 10.1098/rspb.1969.0021. [DOI] [PubMed] [Google Scholar]
- Wright E. M. The origin of the glucose dependent increase in the potential difference across the tortoise small intestine. J Physiol. 1966 Jul;185(2):486–500. doi: 10.1113/jphysiol.1966.sp007998. [DOI] [PMC free article] [PubMed] [Google Scholar]



