Abstract
1. The effect of extracellular pH changes on the voltage—current relation of frog muscle membrane has been studied using intracellular microelectrodes. To reduce the cation conductance of the membrane, potassium in Ringer solution was replaced by rubidium.
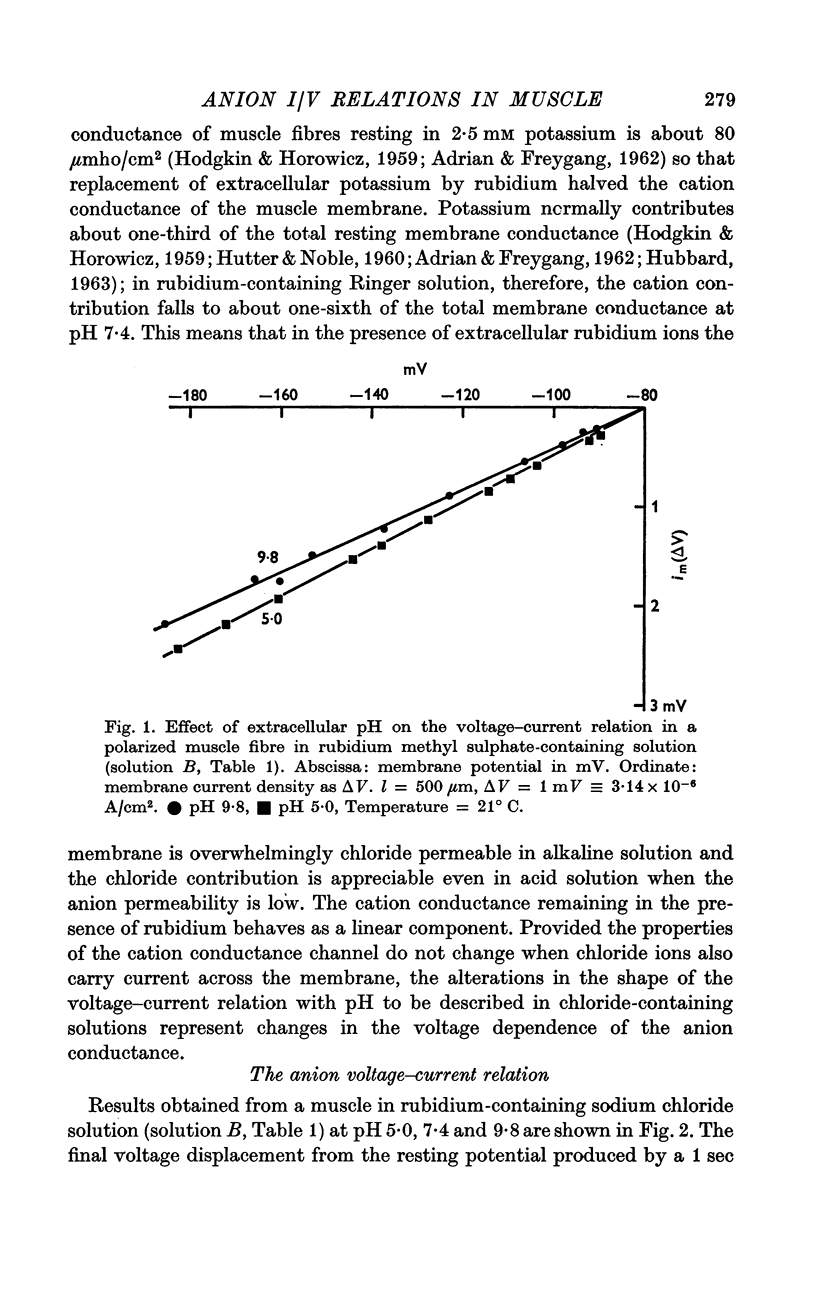
2. When the relatively impermeant methyl sulphate ion replaced extracellular chloride the membrane conductance was low, time independent and little influenced by extracellular pH changes. The voltage—current relation was linear at both high and low pH values.
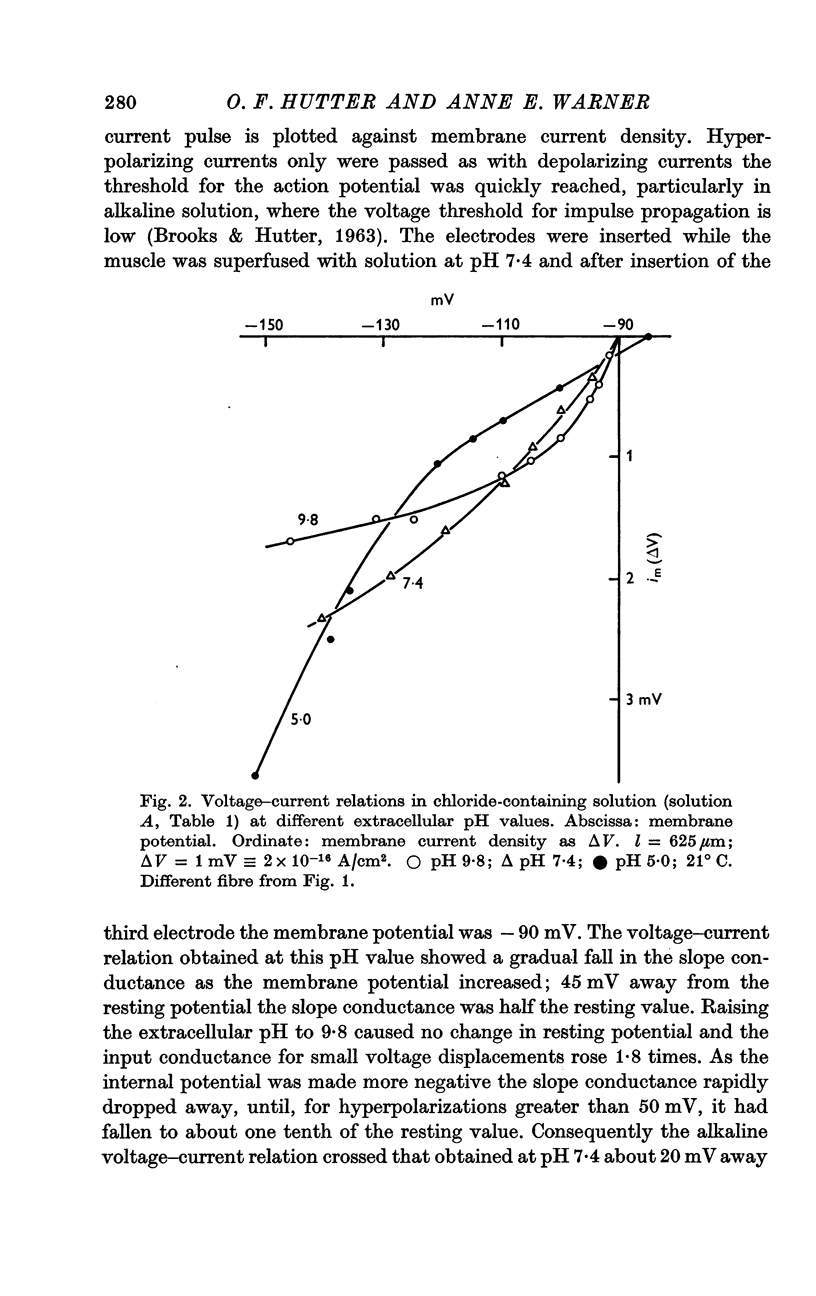
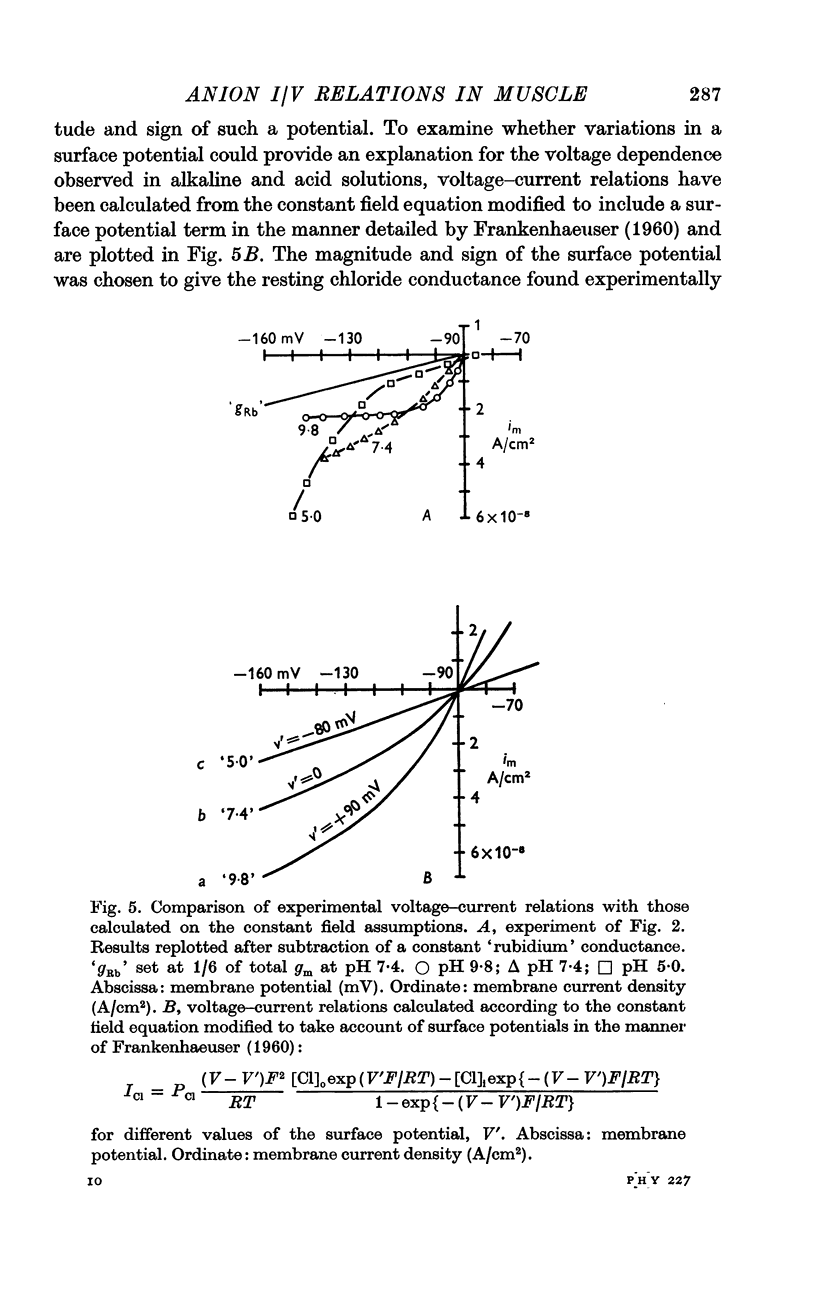
3. In rubidium Ringer solution at pH 7·4 the membrane conductance fell as the inside of the fibre was made more negative, in a manner consistent with the predictions of the constant field theory.
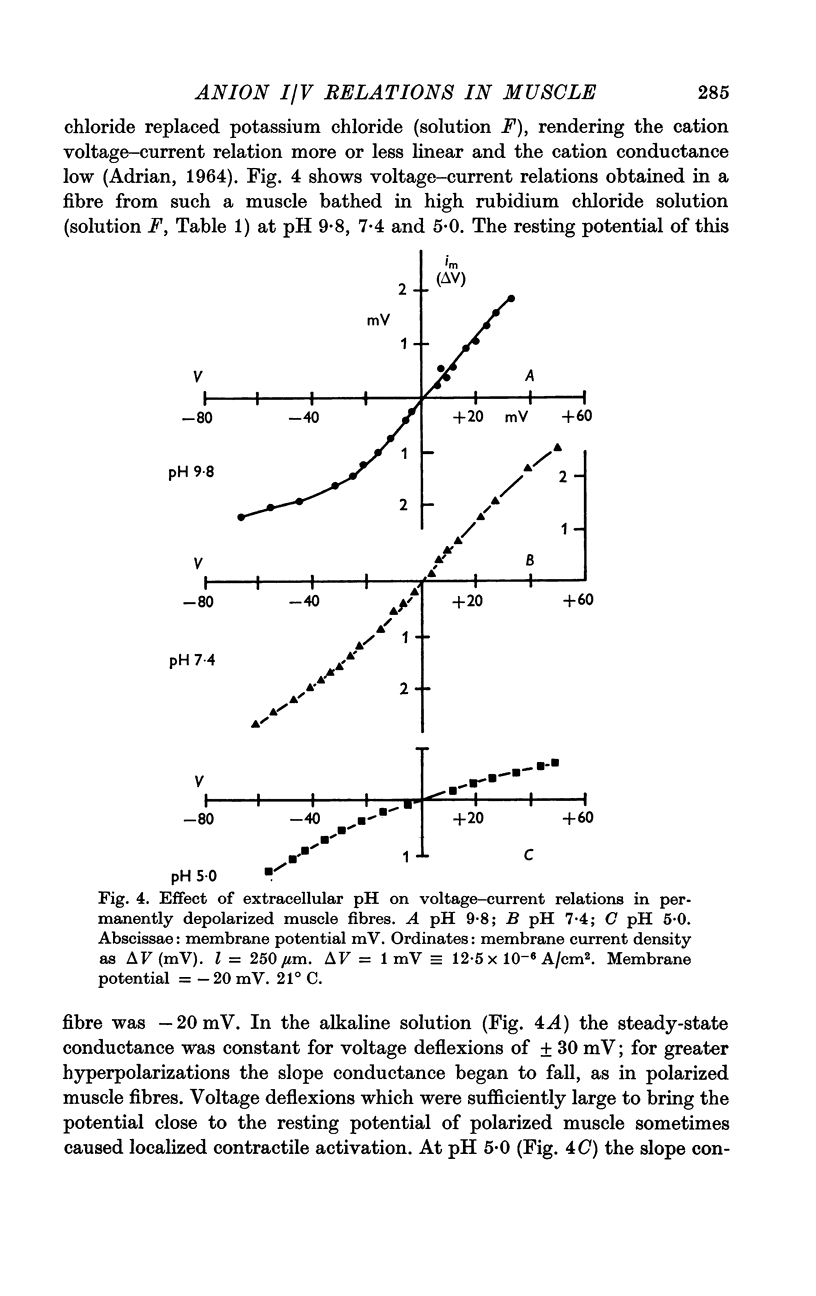
4. At a high pH value (9·8) the resting conductance was high, but it fell steeply as the membrane potential was increased; for large hyperpolarizing voltages the membrane current tended to a limiting value. The voltage—current relation crossed those recorded at pH 7·4 and 5·0.
5. In acid Ringer solution (pH 5·0) the resting membrane conductance was low and remained constant until the membrane potential was hyperpolarized more than 30 mV beyond the resting value; the conductance then rose as the membrane potential was further increased. For large hyperpolarizations the membrane conductance was higher at pH 5·0 than at pH 9·8.
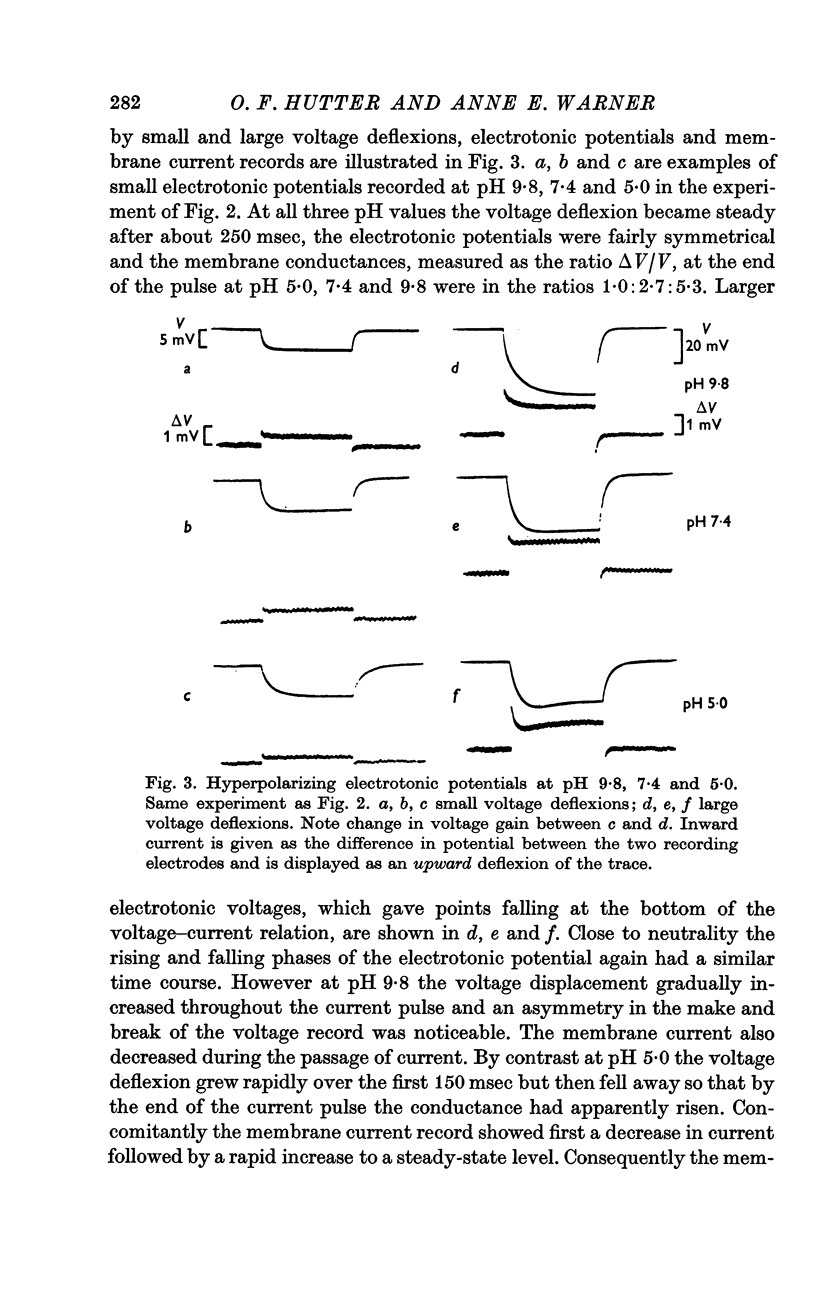
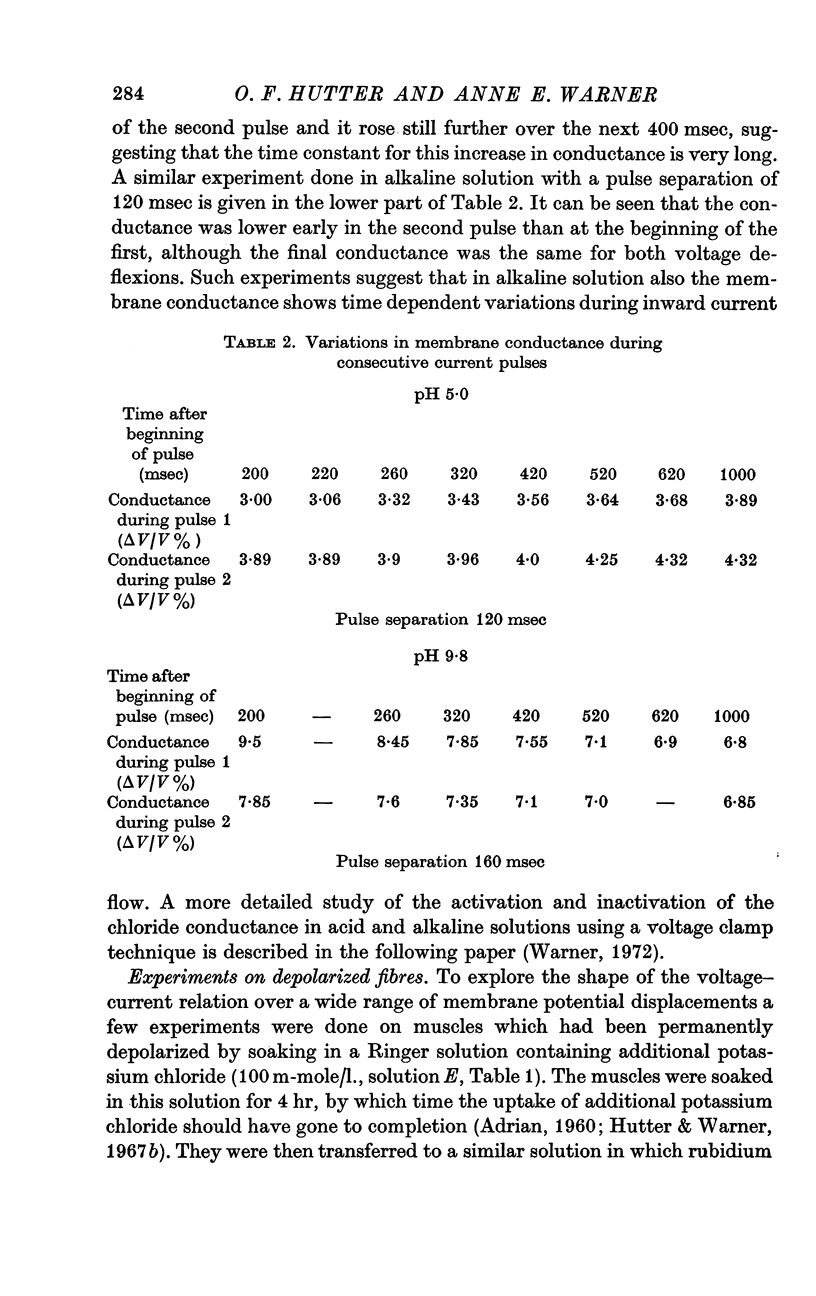
6. Experiments using two successive, identical, constant current pulses suggested that the membrane conductance altered during the passage of current across the membrane; in alkaline solution the conductance fell with time, in acid solution it rose.
7. Because no time or voltage dependence of the membrane conductance was seen in the absence of chloride ions it is inferred that the movements of chloride ions across the muscle membrane are responsible for both the time and voltage dependent alterations in membrane conductance seen at different pH values.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Potassium chloride movement and the membrane potential of frog muscle. J Physiol. 1960 Apr;151:154–185. [PMC free article] [PubMed] [Google Scholar]
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J. P., Walker J. L., Jr Membrane structure and ion permeation. Study of ion exchange membrane structure and function is relevant to analysis of biological ion permeation. Science. 1967 Feb 24;155(3765):965–974. doi: 10.1126/science.155.3765.965. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol. 1969 Mar;53(3):298–310. doi: 10.1085/jgp.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD S. J. The electrical constants and the component conductances of frog skeletal muscle after denervation. J Physiol. 1963 Mar;165:443–456. doi: 10.1113/jphysiol.1963.sp007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. Action of some foreign cations and anions on the chloride permeability of frog muscle. J Physiol. 1967 Apr;189(3):445–460. doi: 10.1113/jphysiol.1967.sp008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The effect of pH on the 36-Cl efflux from frog skeletal muscle. J Physiol. 1967 Apr;189(3):427–443. doi: 10.1113/jphysiol.1967.sp008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H. Sodium, potassium, and chloride fluxes in intercostal muscle from normal goats and goats with hereditary myotonia. J Gen Physiol. 1966 Sep;50(1):89–111. doi: 10.1085/jgp.50.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A. Some Cation Interactions in Muscle. J Gen Physiol. 1961 May 1;44(5):929–962. doi: 10.1085/jgp.44.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F. Membrane ion conductances of frog sartorius fibers as a function of tonicity. Am J Physiol. 1968 Sep;215(3):723–729. doi: 10.1152/ajplegacy.1968.215.3.723. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. E. Kinetic properties of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):291–312. doi: 10.1113/jphysiol.1972.sp010033. [DOI] [PMC free article] [PubMed] [Google Scholar]


