Abstract
1. The effects of pentobarbitone (0·05-0·6 mM in saline solution) on the evoked field potentials of in vitro preparations of guinea-pig olfactory cortex were studied.
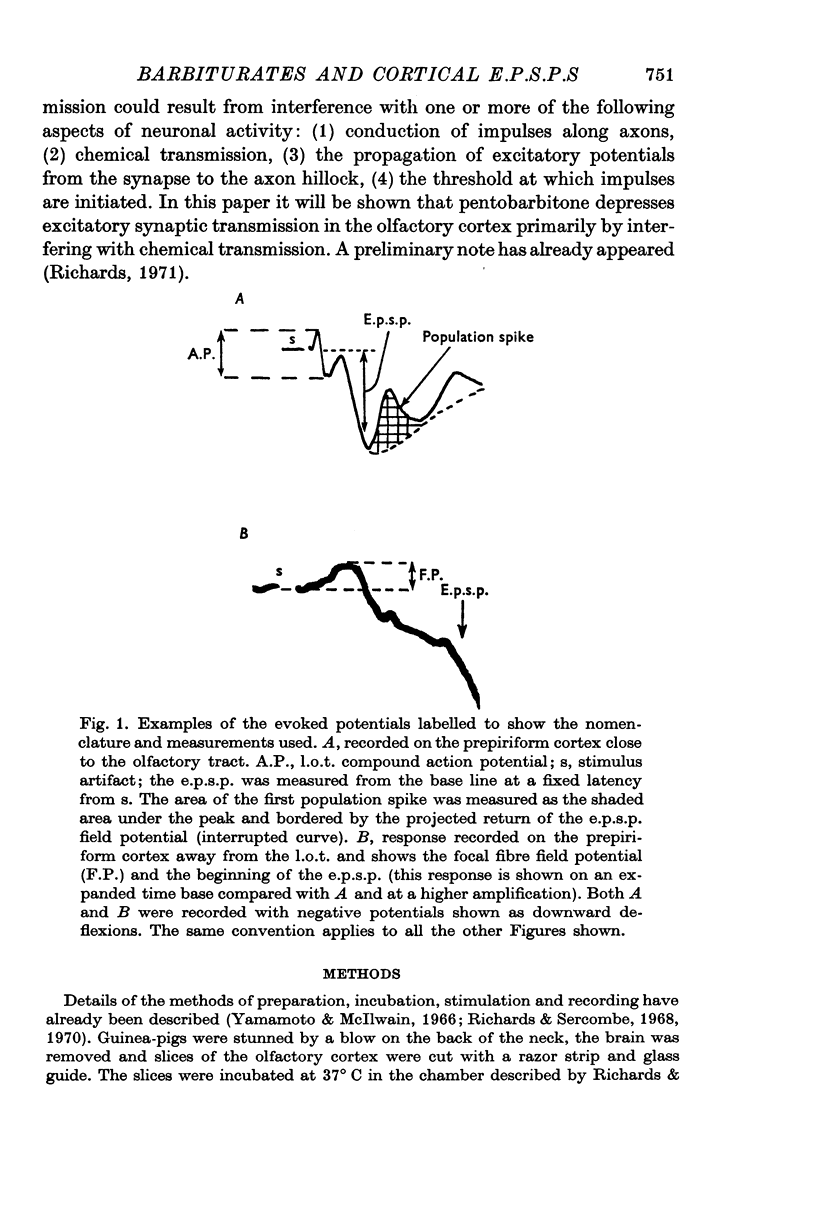
2. The evoked field potentials comprised an initial diphasic wave — the lateral olfactory tract (l.o.t.) compound action potential — followed by a surface negative wave (e.p.s.p) of 1-3 mV amplitude and about 10 msec duration. Superimposed on the negative wave were a number of positive peaks (population spikes).
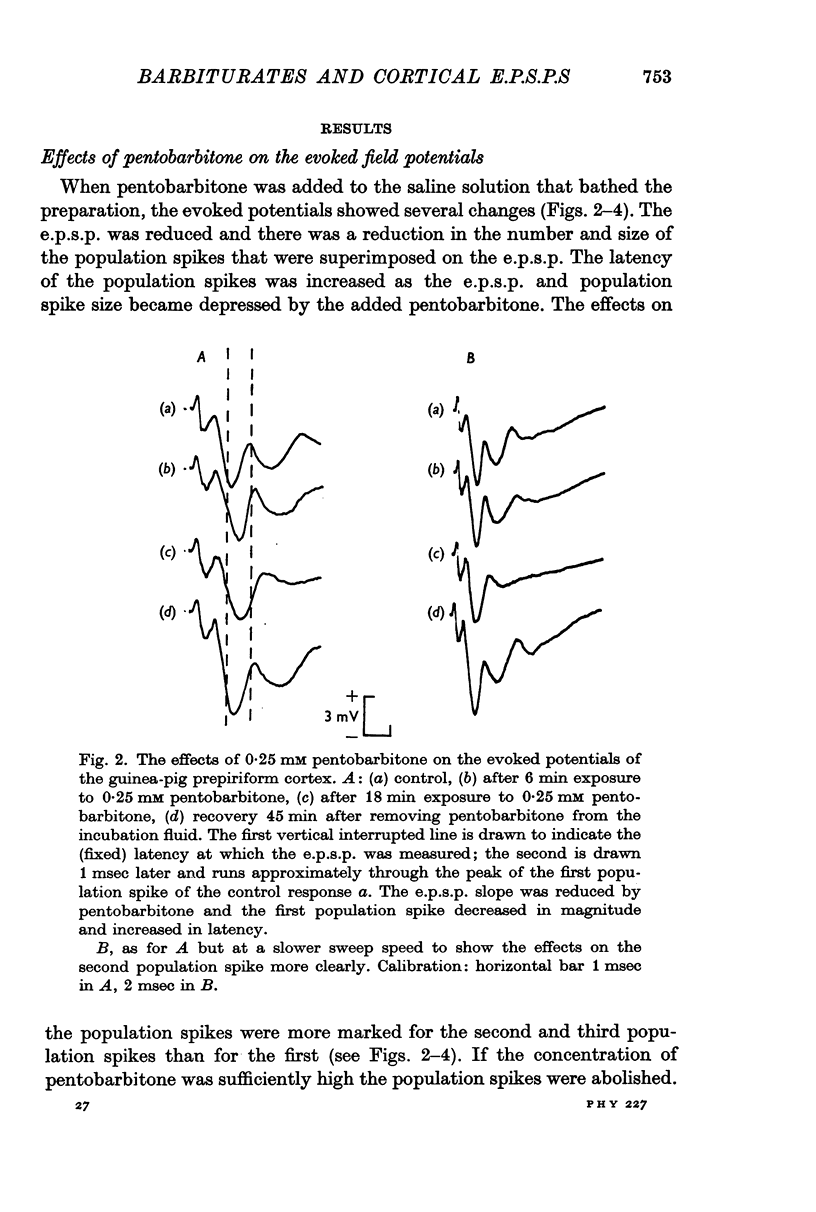
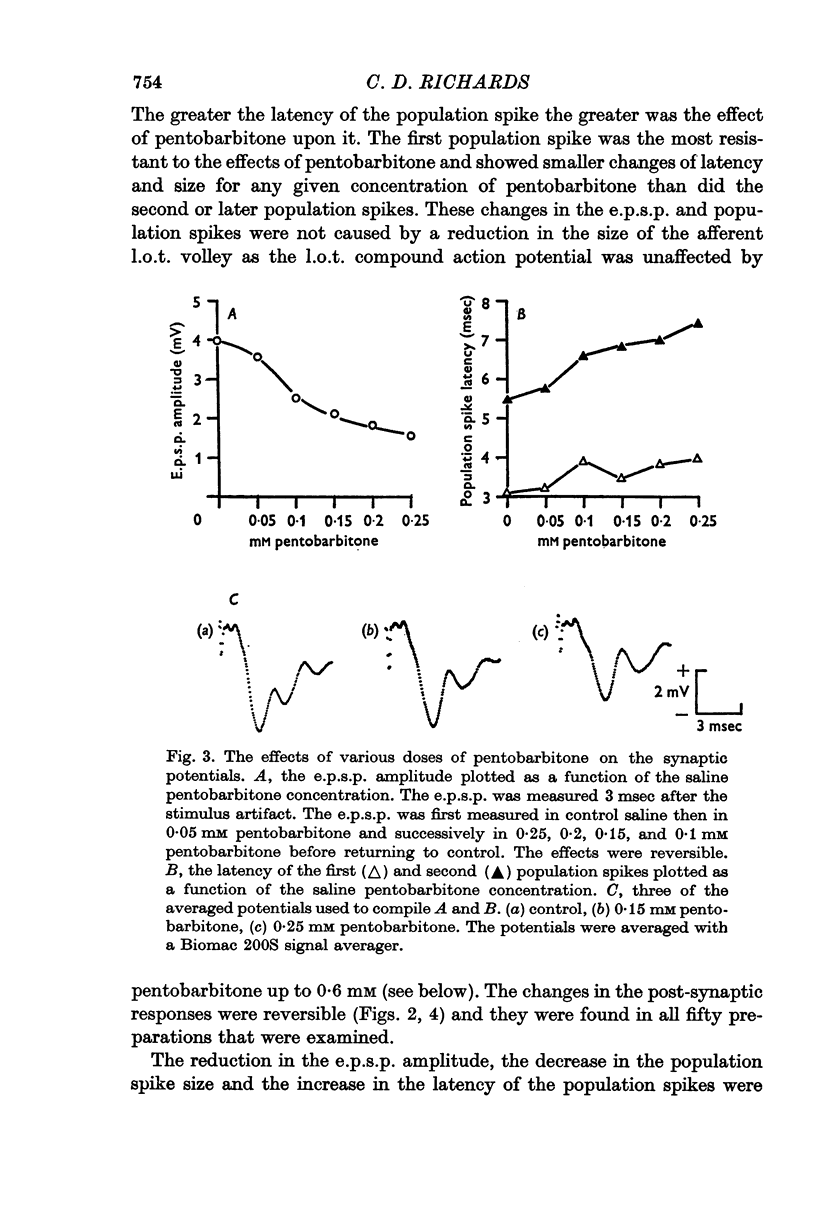
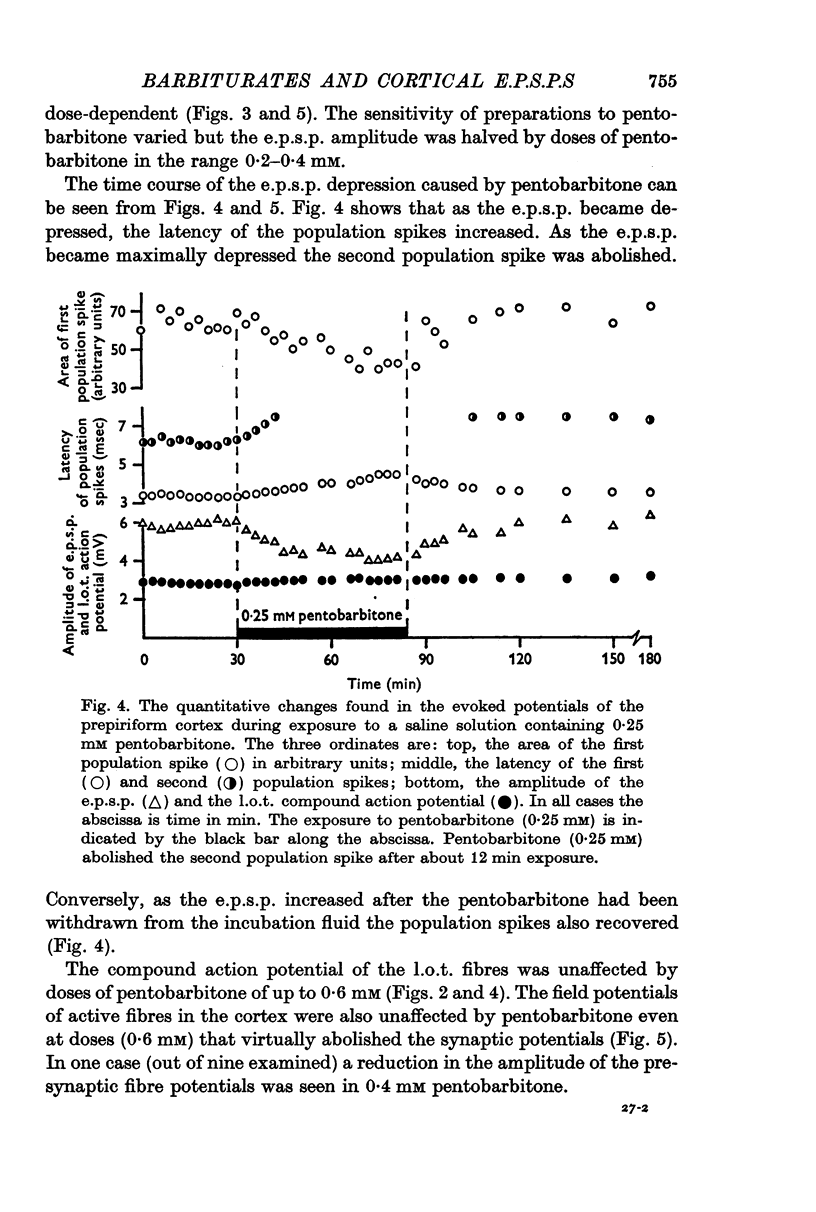
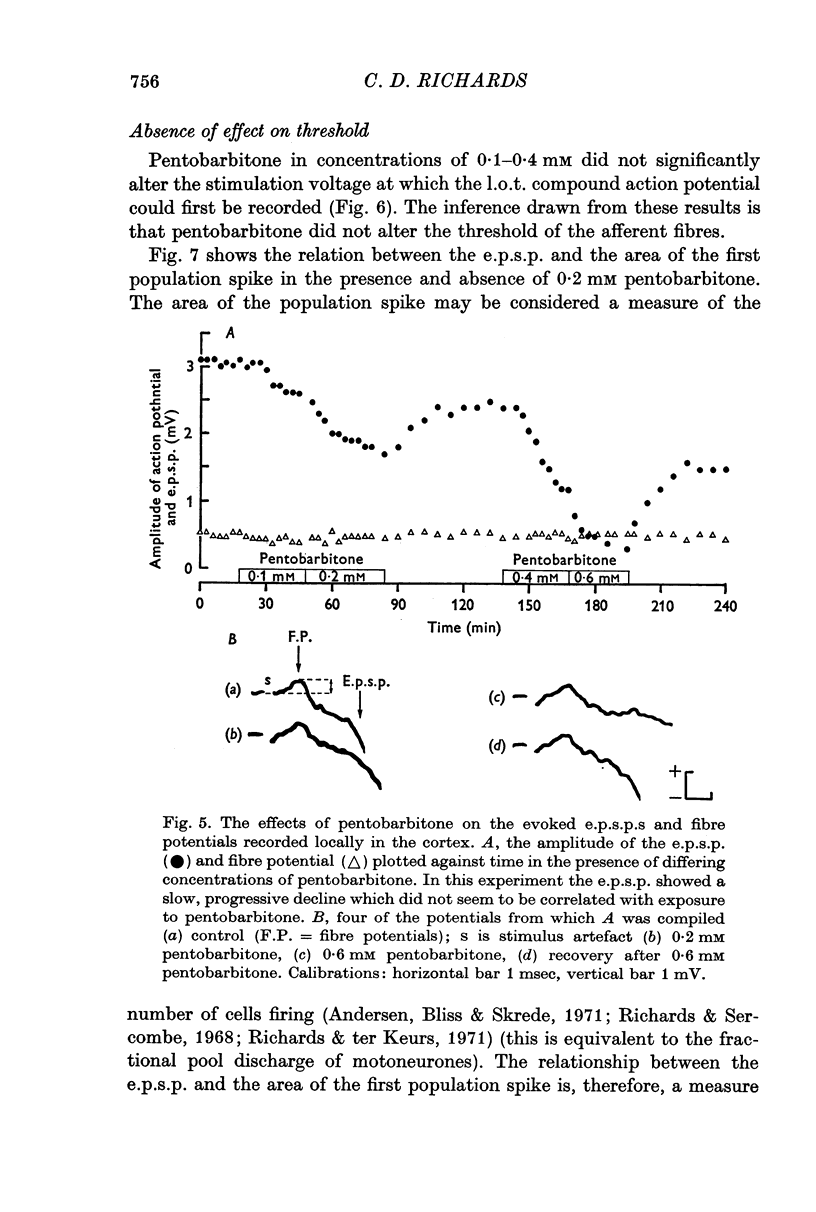
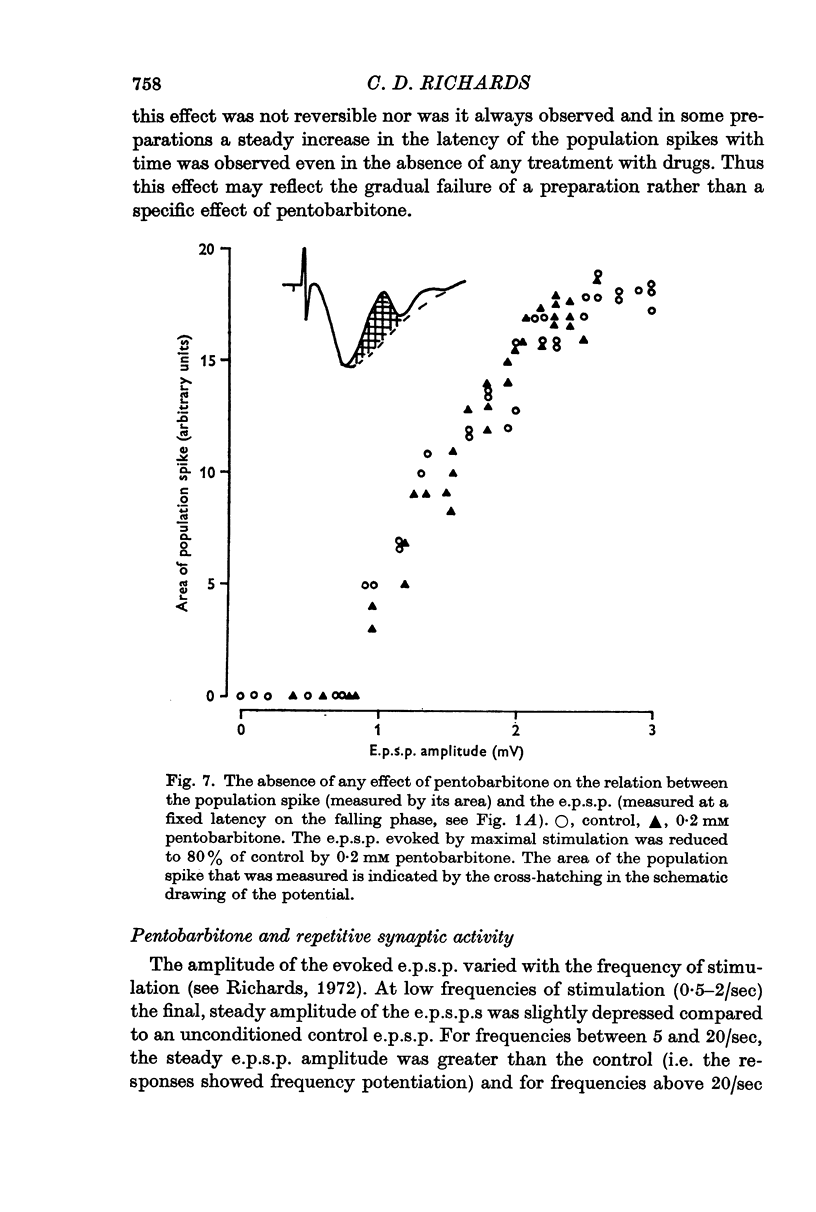
3. Pentobarbitone depressed the e.p.s.p. but not the l.o.t. compound action potential. The number and size of the population spikes were progressively reduced as the e.p.s.p. became depressed, indicating a failure of transmission through the cortical relay. The e.p.s.p. depression increased with increasing concentrations of pentobarbitone.
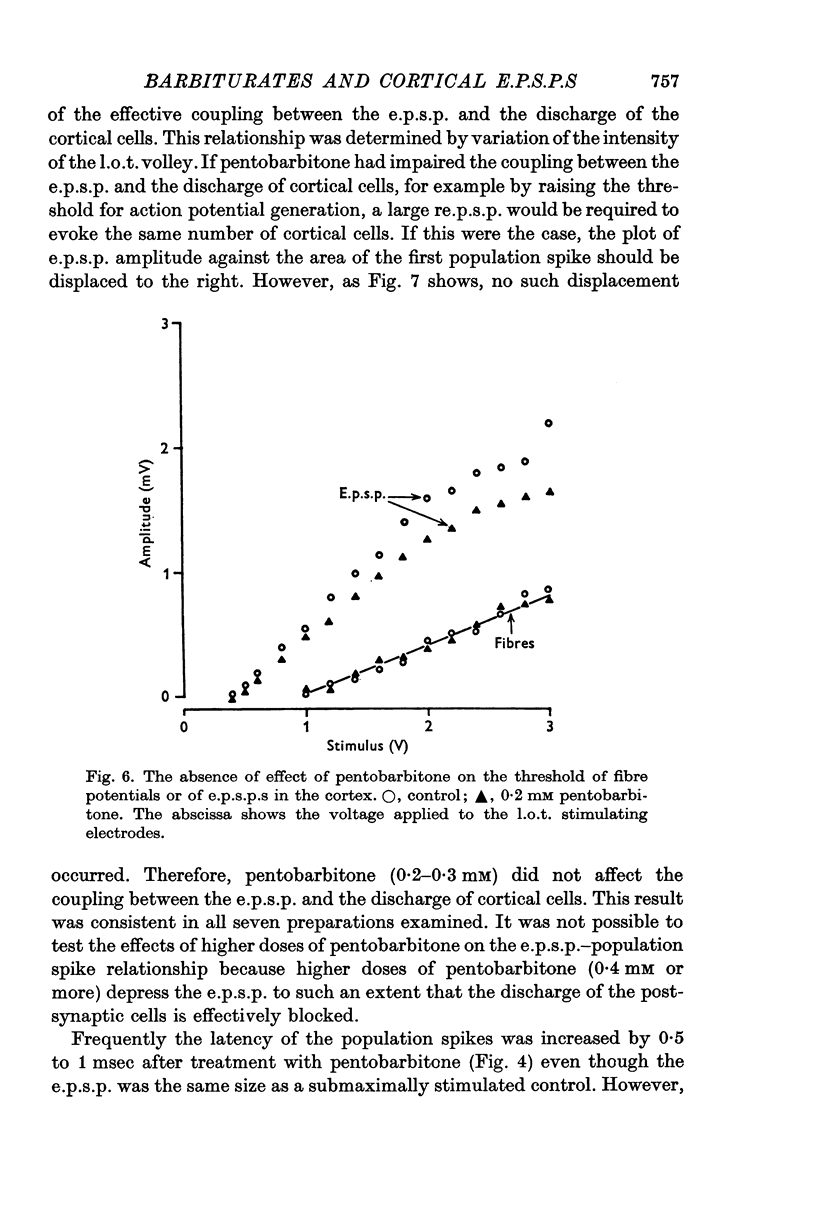
4. Pentobarbitone had no effect on the threshold to electrical stimulation of the l.o.t. fibres or on that of the post-synaptic cells to synaptic excitation.
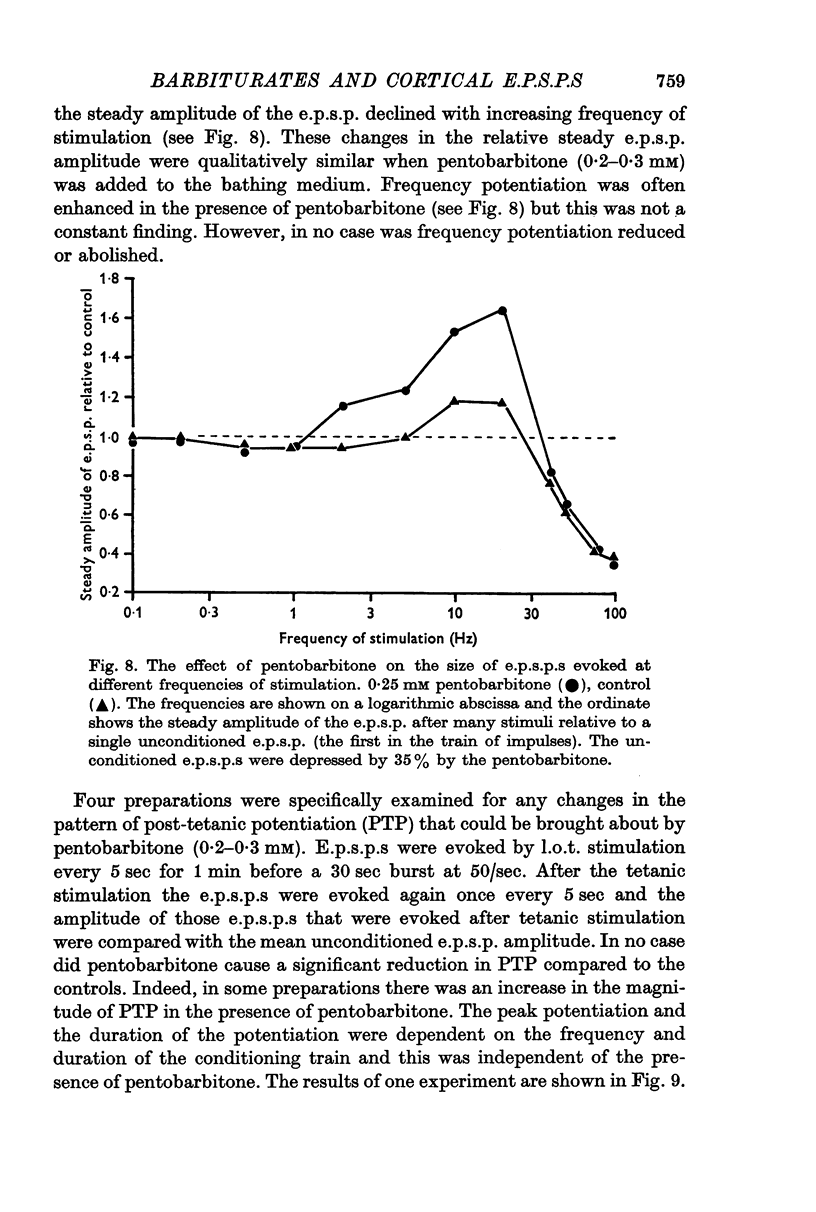
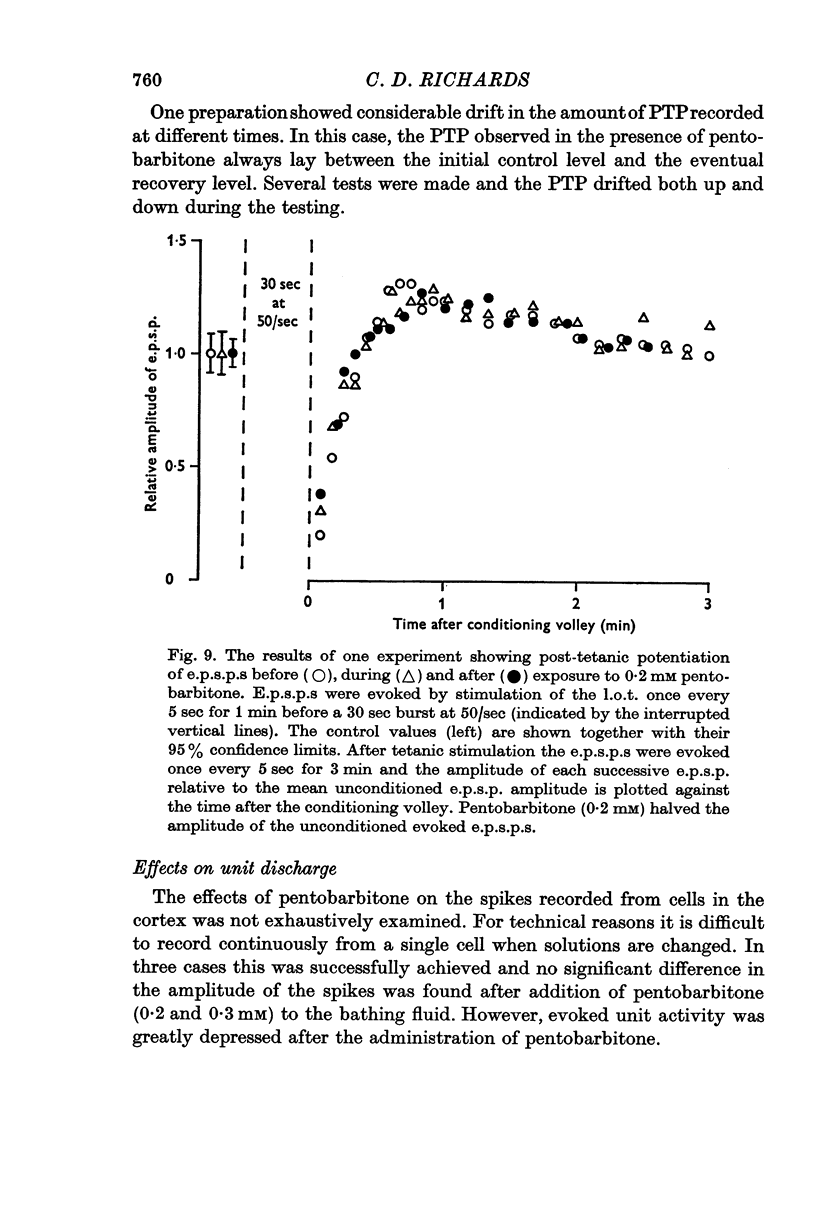
5. Post-tetanic potentiation and frequency potentiation were either of normal magnitude or were enhanced in the presence of 0·2-0·3 mM pentobarbitone.
6. It is concluded that pentobarbitone probably reduces the output of transmitter from the presynaptic nerve terminals of the olfactory cortex and that this mechanism could be the basis of the depressant action of the barbiturates.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- BRODIE B. B., MARK L. C. The fate of thiopental in man and a method for its estimation in biological material. J Pharmacol Exp Ther. 1950 Jan;98(1):85–96. [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G. B., Ota M. Blockade of the reticulospinal inhibitory pathway by anaesthetic agents. Br J Pharmacol. 1971 Jul;42(3):328–342. doi: 10.1111/j.1476-5381.1971.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- Narth C. S. An inexpensive field effect transistor preamplifier, for use with entracellular micro-electrodes. J Physiol. 1969 Feb;200(2):102P–103P. [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. Potentiation and depression of synaptic transmission in the olfactory cortex of the guinea-pig. J Physiol. 1972 Apr;222(1):209–231. doi: 10.1113/jphysiol.1972.sp009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J Physiol. 1970 Dec;211(3):571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Electrical activity observed in guinea-pig olfactory cortex maintained in vitro. J Physiol. 1968 Aug;197(3):667–683. doi: 10.1113/jphysiol.1968.sp008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Ter Keurs W. J. The effects of tetrodotoxin on the evoked potentials of the guinea-pig prepiriform cortex. Brain Res. 1971 Mar 5;26(2):446–449. [PubMed] [Google Scholar]
- Richards C. D. The selective depression of evoked cortical EPSPs by pentobarbitone. J Physiol. 1971;217 (Suppl):41P–43P. [PubMed] [Google Scholar]
- SOMJEN G. G. Effects of ether and thiopental on spinal presynaptic terminals. J Pharmacol Exp Ther. 1963 Jun;140:396–402. [PubMed] [Google Scholar]
- SOMJEN G. G., GILL M. The mechanism of the blockade of synaptic transmission in the mammalian spinal cord by diethyl ether and by thiopental. J Pharmacol Exp Ther. 1963 Apr;140:19–30. [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- WALL P. D., JOHNSON A. R. Changes associated with post-tetanic potentiation of a monosynaptic reflex. J Neurophysiol. 1958 Mar;21(2):148–158. doi: 10.1152/jn.1958.21.2.148. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N., Esplin D. W., Zablocka B. Criteria for assessing effects of drugs on postsynaptic inhibition. Arch Int Pharmacodyn Ther. 1968 Feb;171(2):385–393. [PubMed] [Google Scholar]
- Yamamoto C., McIlwain H. Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. J Neurochem. 1966 Dec;13(12):1333–1343. doi: 10.1111/j.1471-4159.1966.tb04296.x. [DOI] [PubMed] [Google Scholar]


