Abstract
1. The responses of single cells to mechanical movements of individual whiskers have been recorded from the ventro-basal complex of the thalamus, in rats under urethane or barbiturate anaesthesia.
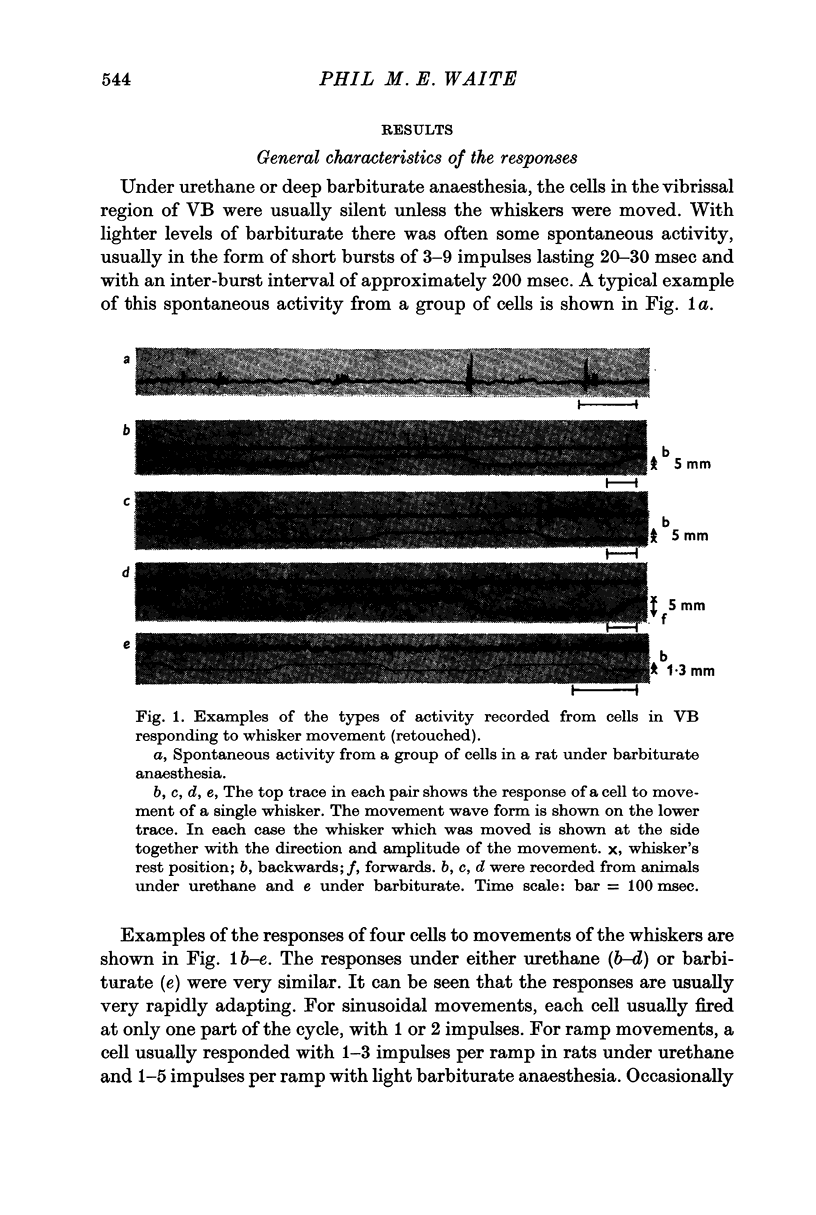
2. With ramp-shaped displacements of a whisker above a critical velocity, the cells gave a short latency response of 1-5 impulses, while with sinusoidal movement (1-35 Hz) they usually responded with 1-2 impulses per cycle.
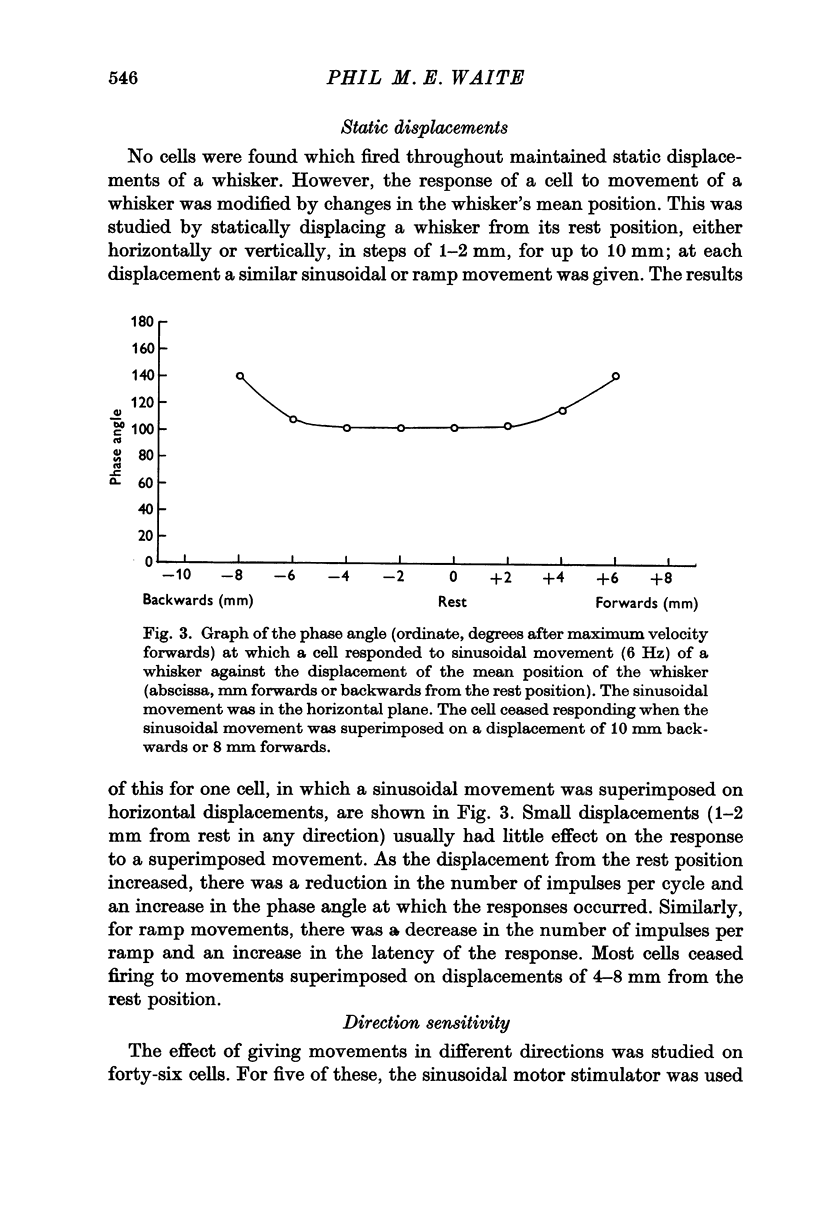
3. The cells did not respond to maintained deflexions of a whisker. Small static displacements did not modify the response to a superimposed movement; larger static displacements reduced or abolished the response.
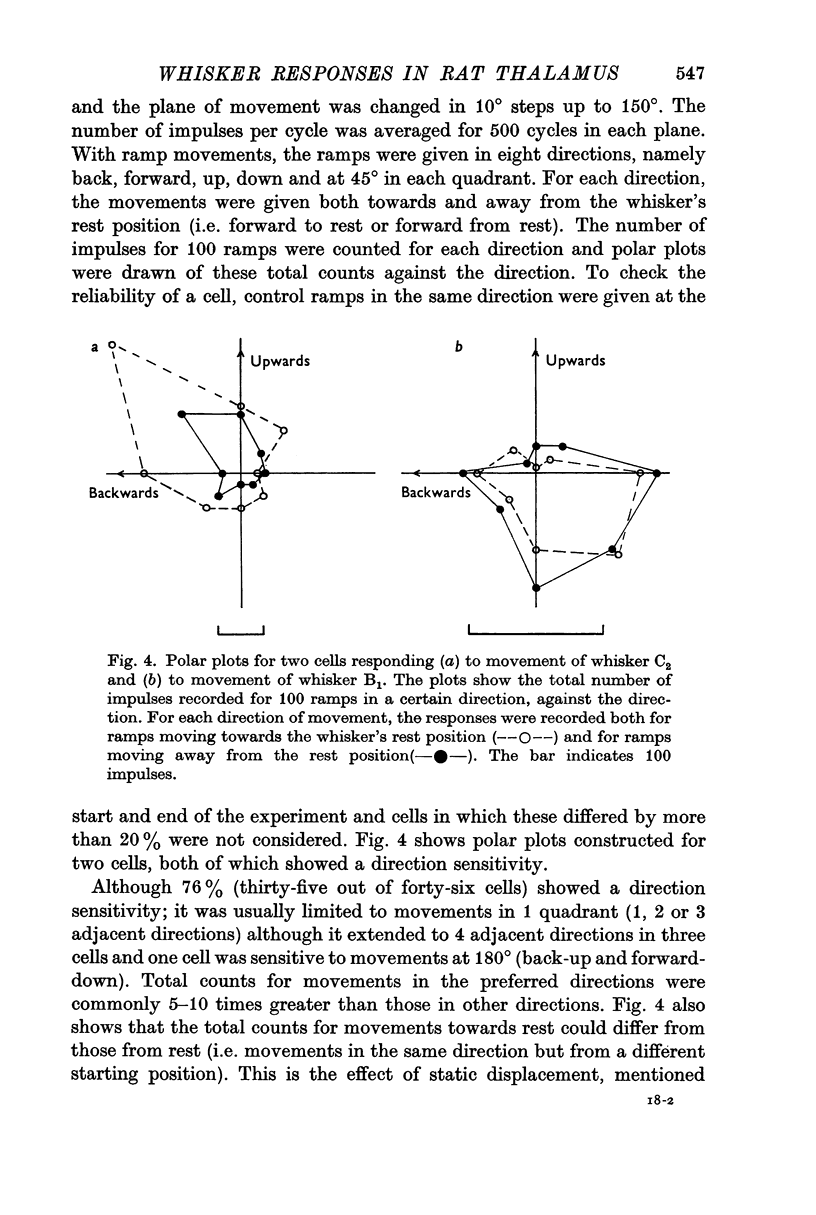
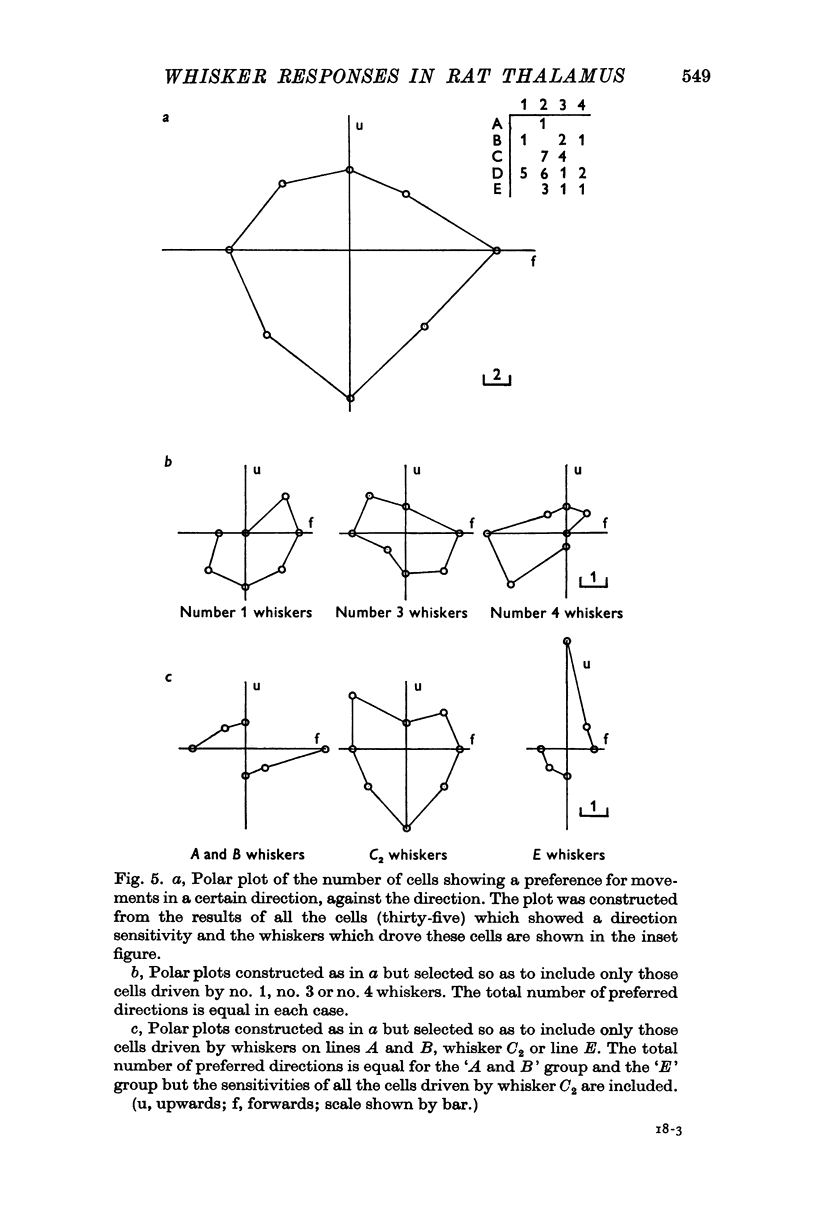
4. Three-quarters of the cells were found to be particularly sensitive to movements in one quadrant (90° or less). For any one cell, there was no obvious relationship between the most sensitive direction and the position of the whisker on the face.
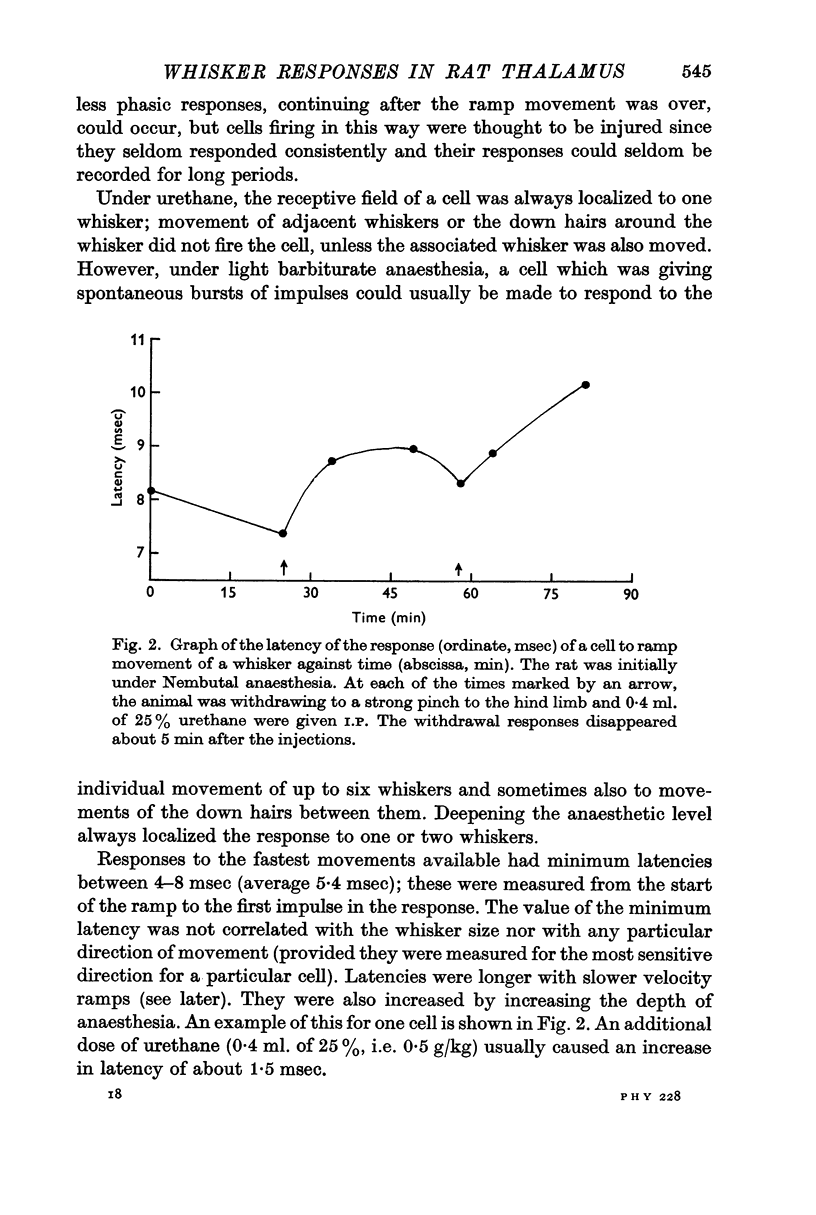
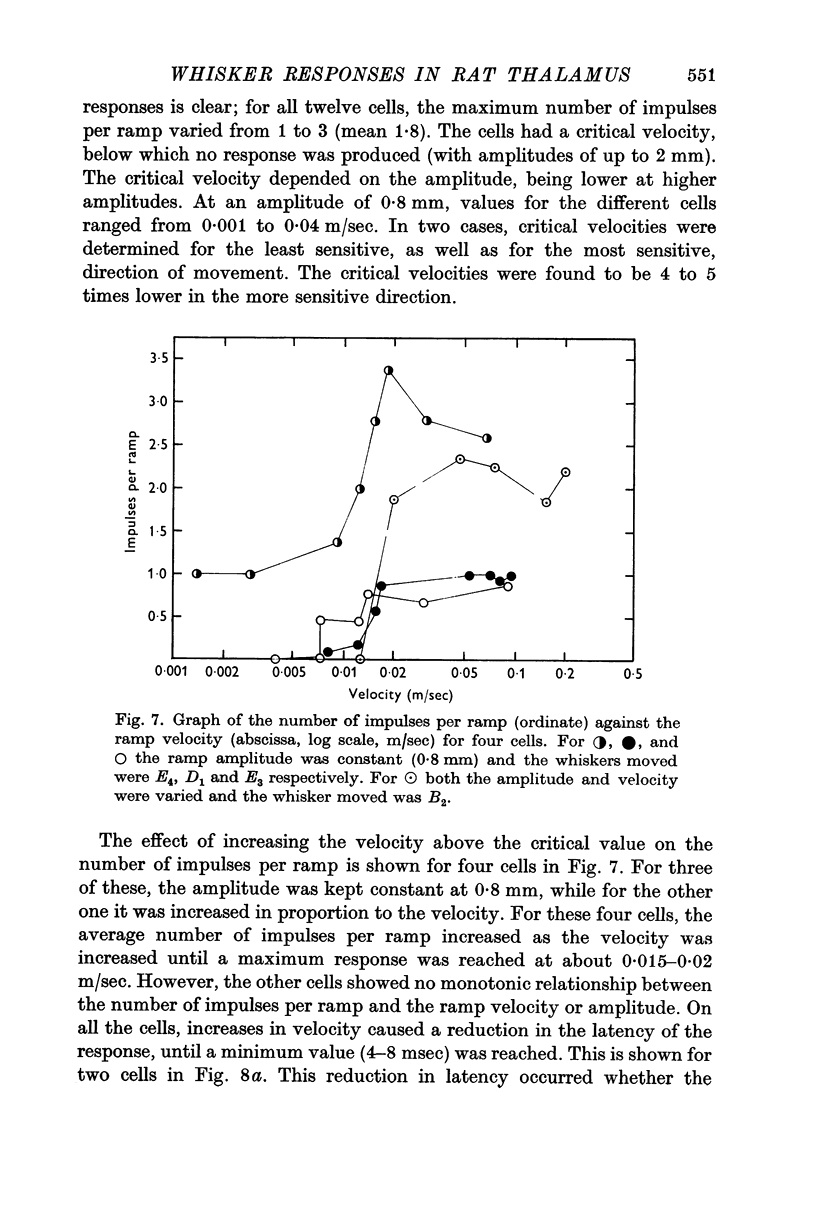
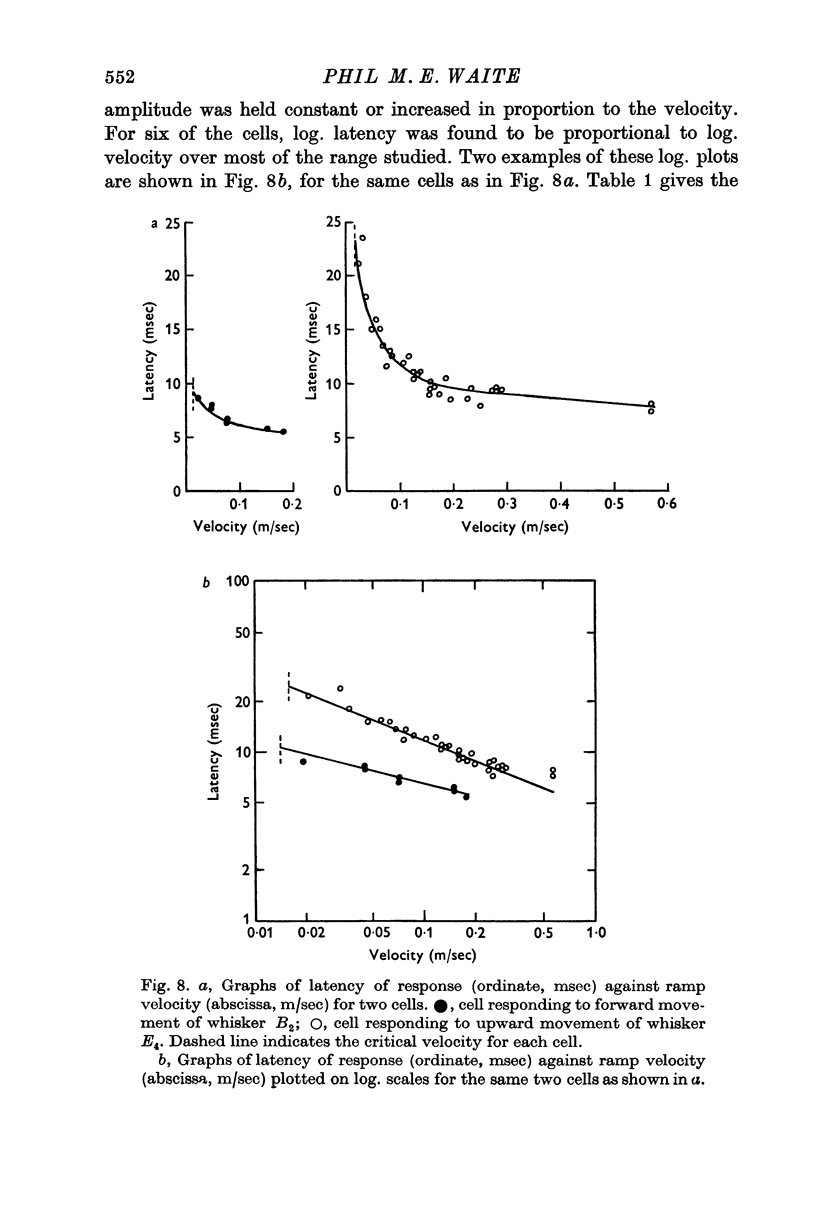
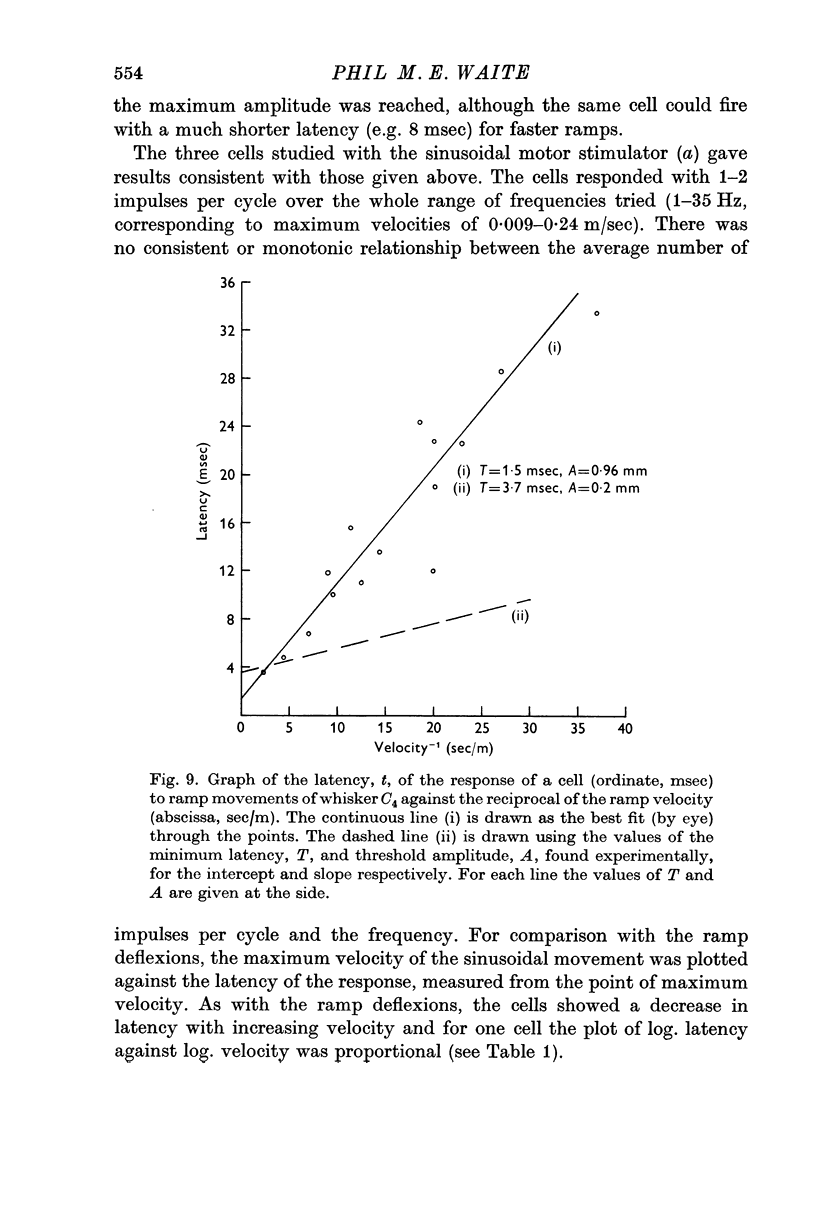
5. The ramp amplitude appeared to have little effect on the response. However, increases in ramp velocity decreased the response latency and, in some cells, increased the number of impulses per ramp.
6. Other studies have shown that most afferent nerve fibres from whiskers give slowly adapting responses and the possible modification of these thalamic responses, by anaesthesia, is discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albe-Fessard D. Organization of somatic central projections. Contrib Sens Physiol. 1967;2:101–167. doi: 10.1016/b978-1-4831-6749-7.50009-x. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Davidson N. The projection of afferent pathways on the thalamus of the rat. J Comp Neurol. 1965 Jun;124(3):377–390. doi: 10.1002/cne.901240308. [DOI] [PubMed] [Google Scholar]
- Fitzgerald O. Discharges from the sensory organs of the cat's vibrissae and the modification in their activity by ions. J Physiol. 1940 May 14;98(2):163–178. doi: 10.1113/jphysiol.1940.sp003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., LANDGREN S., SEED W. A. The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol. 1961 Oct;158:544–559. doi: 10.1113/jphysiol.1961.sp006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGLUND G., LINDBLOM U. The discharge in single touch receptors elicited by defined mechanical stimuli. Acta Physiol Scand. 1961 Jun;52:108–119. doi: 10.1111/j.1748-1716.1961.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Hahn J. F. Stimulus-response relationships in first-order sensory fibres from cat vibrissae. J Physiol. 1971 Feb;213(1):215–226. doi: 10.1113/jphysiol.1971.sp009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR F. W., LYSAK W. R. SOMATOTOPIC ORGANIZATION OF TRIGEMINAL-GANGLION NEURONES. Arch Neurol. 1964 Dec;11:593–602. doi: 10.1001/archneur.1964.00460240025003. [DOI] [PubMed] [Google Scholar]
- KRUGER L., MICHEL F. Reinterpretation of the representation of pain based on physiological excitation of single neurons in the trigeminal sensory complex. Exp Neurol. 1962 Feb;5:157–178. doi: 10.1016/0014-4886(62)90031-6. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., DAVIES P. W., BERMAN A. L. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957 Jul;20(4):374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Central nervous mechanisms subserving position sense and kinesthesis. Bull Johns Hopkins Hosp. 1959 Oct;105:173–200. [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959 Oct;105:201–232. [PubMed] [Google Scholar]
- Nilsson B. Y., Skoglund C. R. The tactile hairs on the cat's foreleg. Acta Physiol Scand. 1965 Dec;65(4):364–369. doi: 10.1111/j.1748-1716.1965.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Nord S. G. Receptor field characteristics of single cells in the rat spinal trigeminal complex. Exp Neurol. 1968 Jun;21(2):236–243. doi: 10.1016/0014-4886(68)90142-8. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., MOUNTCASTLE V. B. THE FUNCTIONAL PROPERTIES OF VENTROBASAL THALAMIC NEURONSSTUDIED IN UNANESTHETIZED MONKEYS. J Neurophysiol. 1963 Sep;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- ROSE J. E., MOUNTCASTLE V. B. Activity of single neurons in the tactile thalamic region of the cat in response to a transient peripheral stimulus. Bull Johns Hopkins Hosp. 1954 May;94(5):238–282. [PubMed] [Google Scholar]
- TORVIK A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956 Nov;106(1):51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- TOWE A. L., KENNEDY T. T. Response of cortical neurons to variation of stimulus intensity and locus. Exp Neurol. 1961 Jun;3:570–587. doi: 10.1016/s0014-4886(61)80006-x. [DOI] [PubMed] [Google Scholar]
- WOLBARSHT M. L., DETHIER V. G. Electrical activity in the chemoreceptors of the blowfly. I. Responses to chemical and mechanical stimulation. J Gen Physiol. 1958 Nov 20;42(2):393–412. doi: 10.1085/jgp.42.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]




