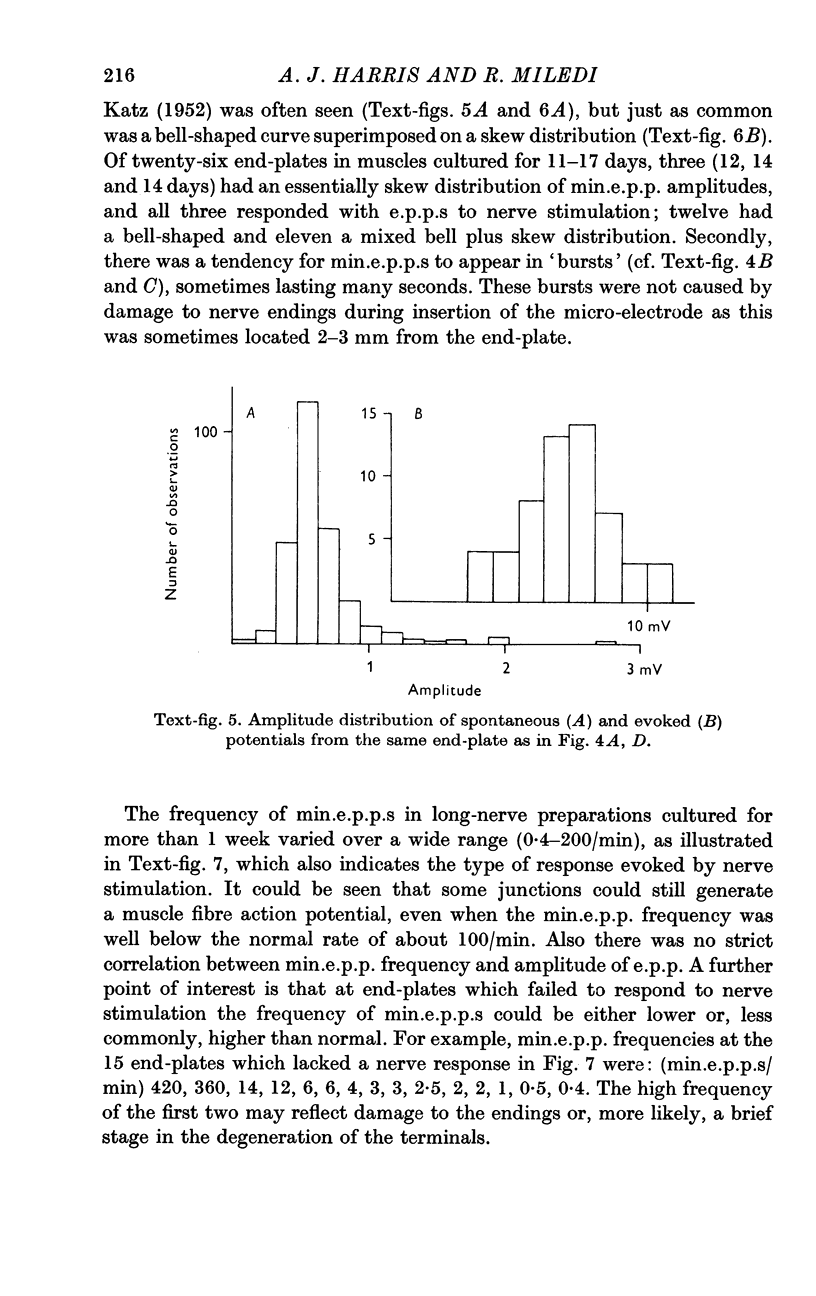
Abstract
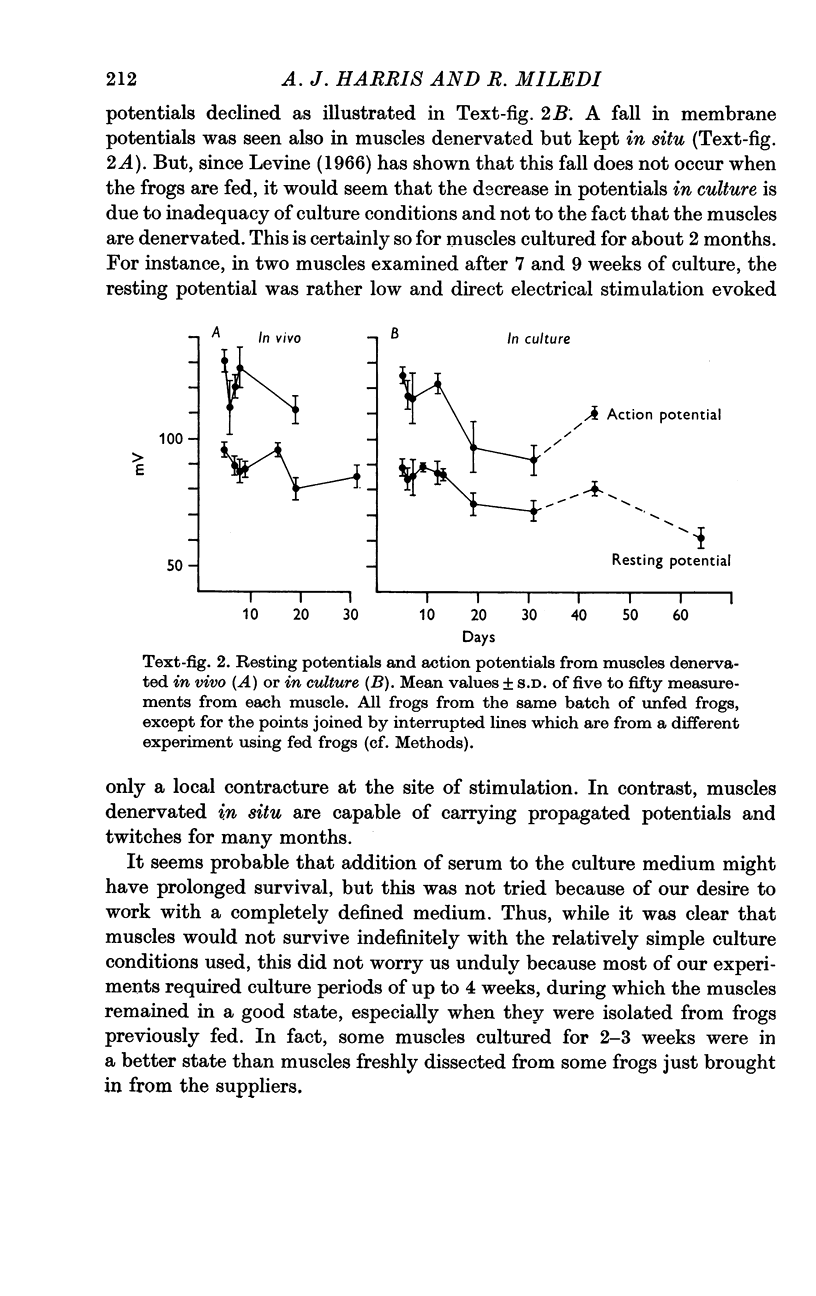
1. Frog muscles are isolated and maintained in organ culture conditions for periods of up to 2 months. During the first 2 weeks, muscle fibres have normal resting membrane and action potentials. Subsequently the potentials decline in amplitude.
2. Slow muscle fibres also survive in culture and retain their ability to give maintained contractures.
3. Muscle sensory receptors continue to function in culture until the axon terminals degenerate at about 2 weeks.
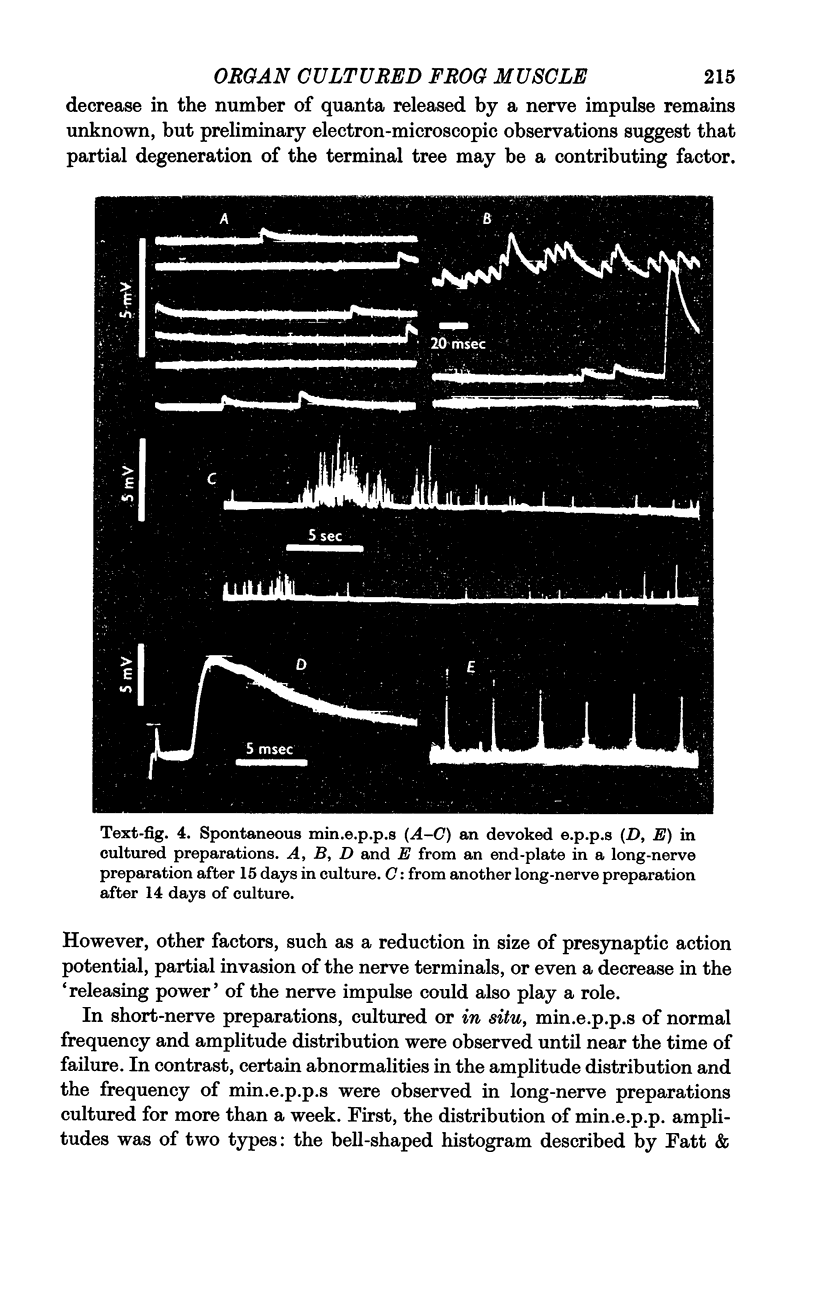
4. Neuromuscular transmission is normal during the first few days of culture, after which the motor endings degenerate. Transmission persists longer (up to 17 days) if a long segment of nerve is left attached to the muscle. With short-nerve preparations failure of transmission in vivo occurs at about the same time as in culture. With long-nerve preparations failure of transmission is delayed even further in culture.
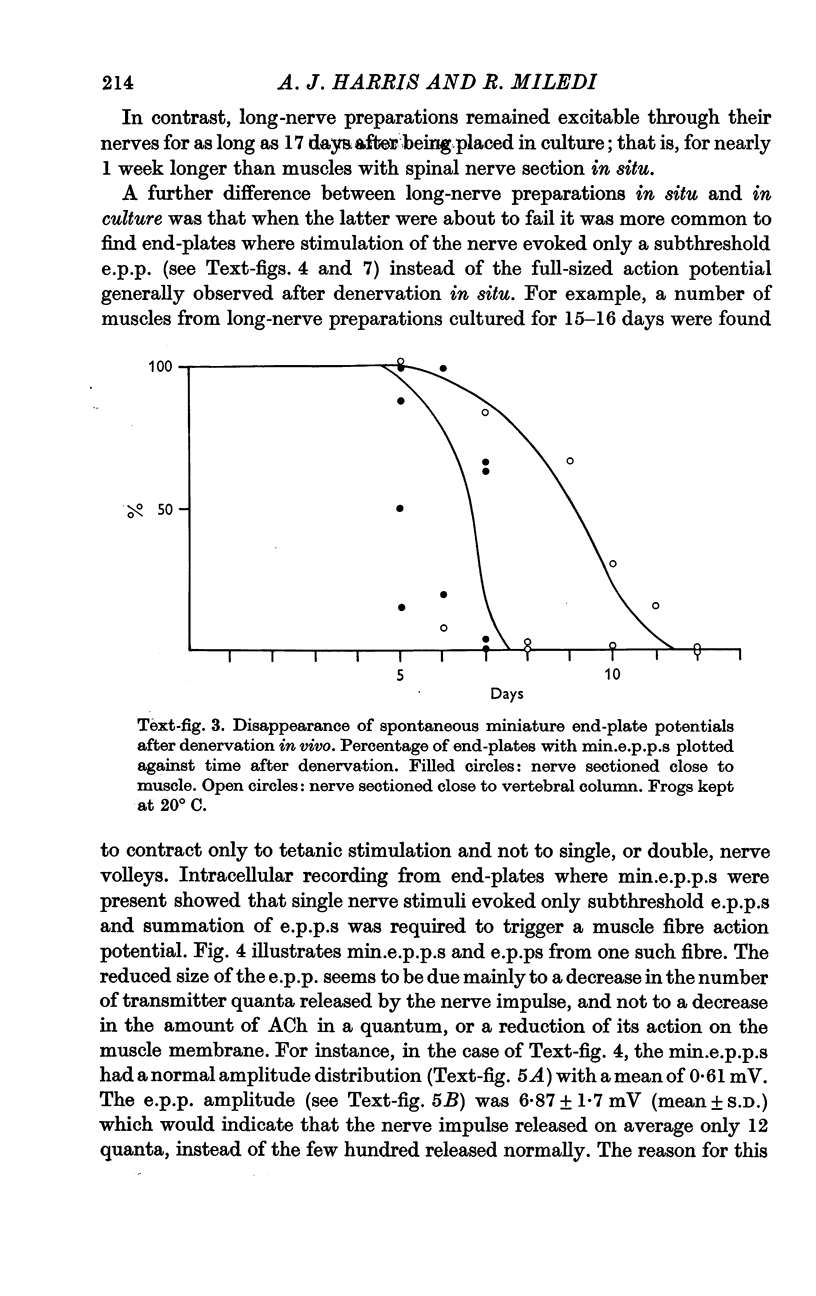
5. In short-nerve preparations miniature end-plate potentials disappear, in general, at about the time that transmission fails. In long-nerve preparations some end-plates continue to have miniature end-plate potential activity for a short time after nerve impulses cease to evoke any response; but eventually miniature potential activity disappears from all end-plates.
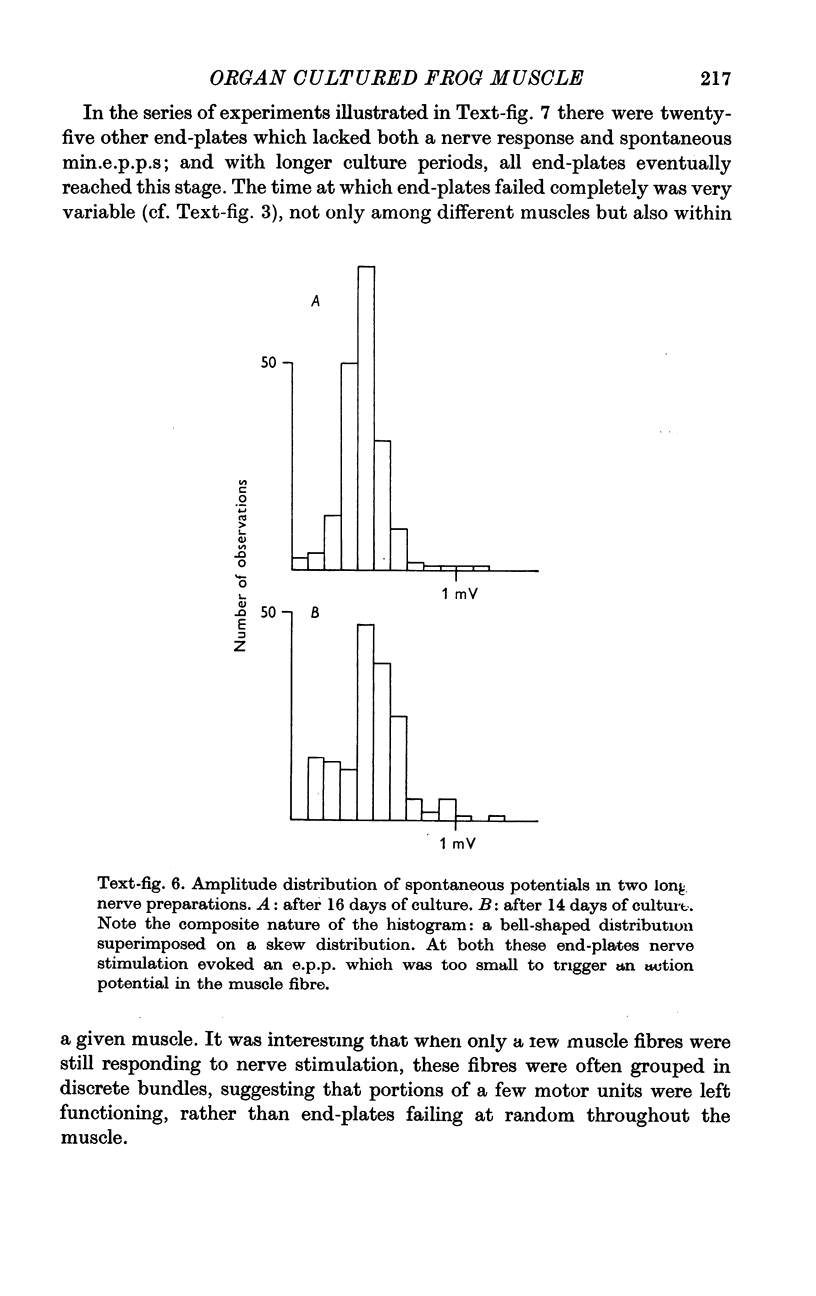
6. After a few days of electrical silence, miniature end-plate potentials reappear at some of the denervated end-plates. The proportion of denervated end-plates which show miniature end-plate potentials in culture is smaller than in muscles denervated in situ.
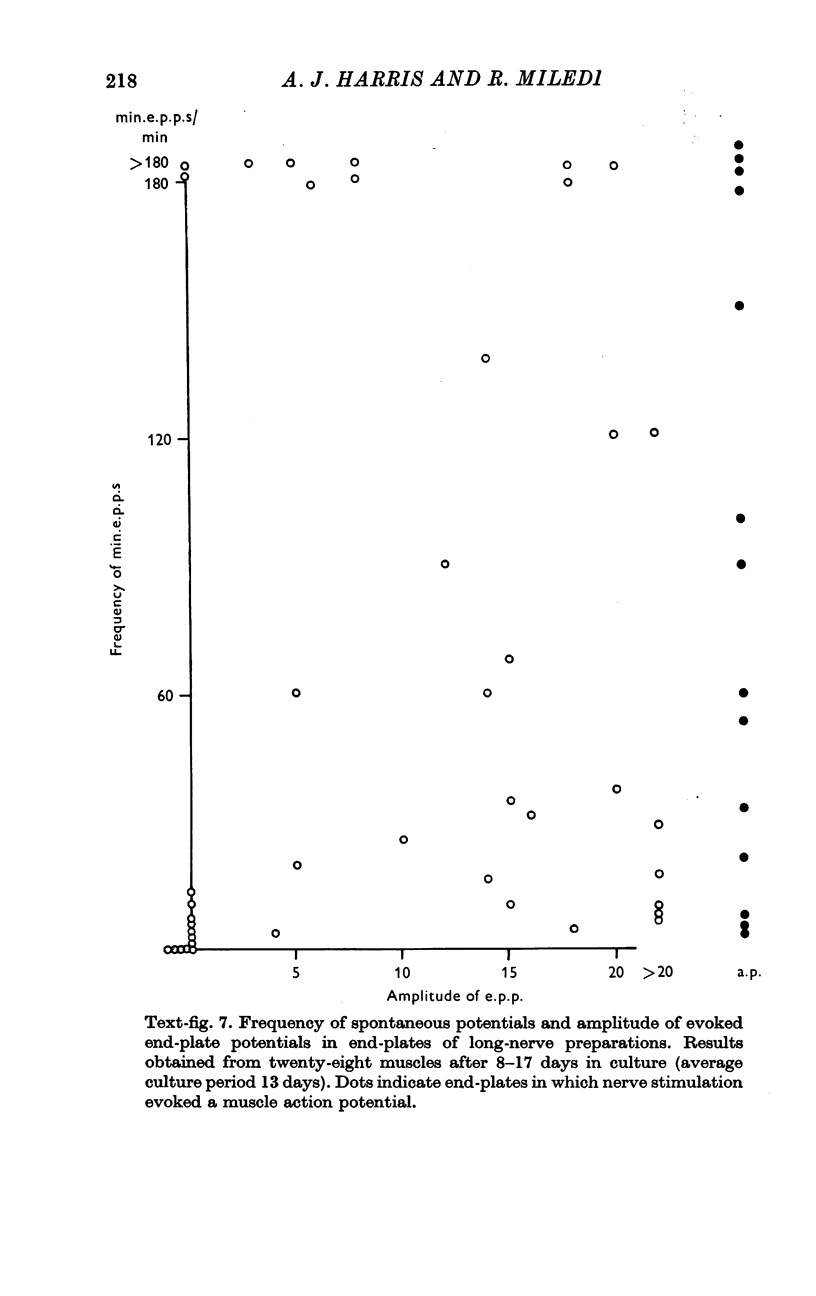
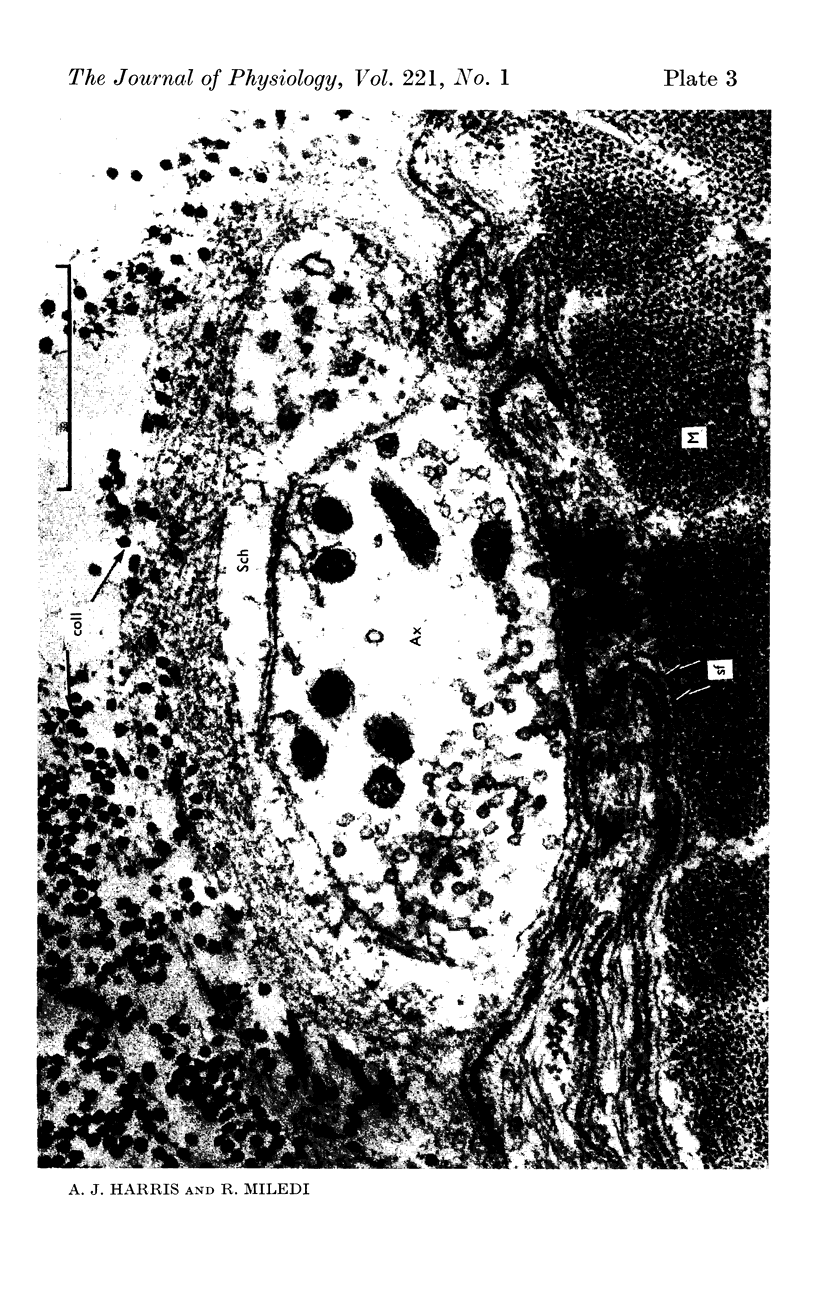
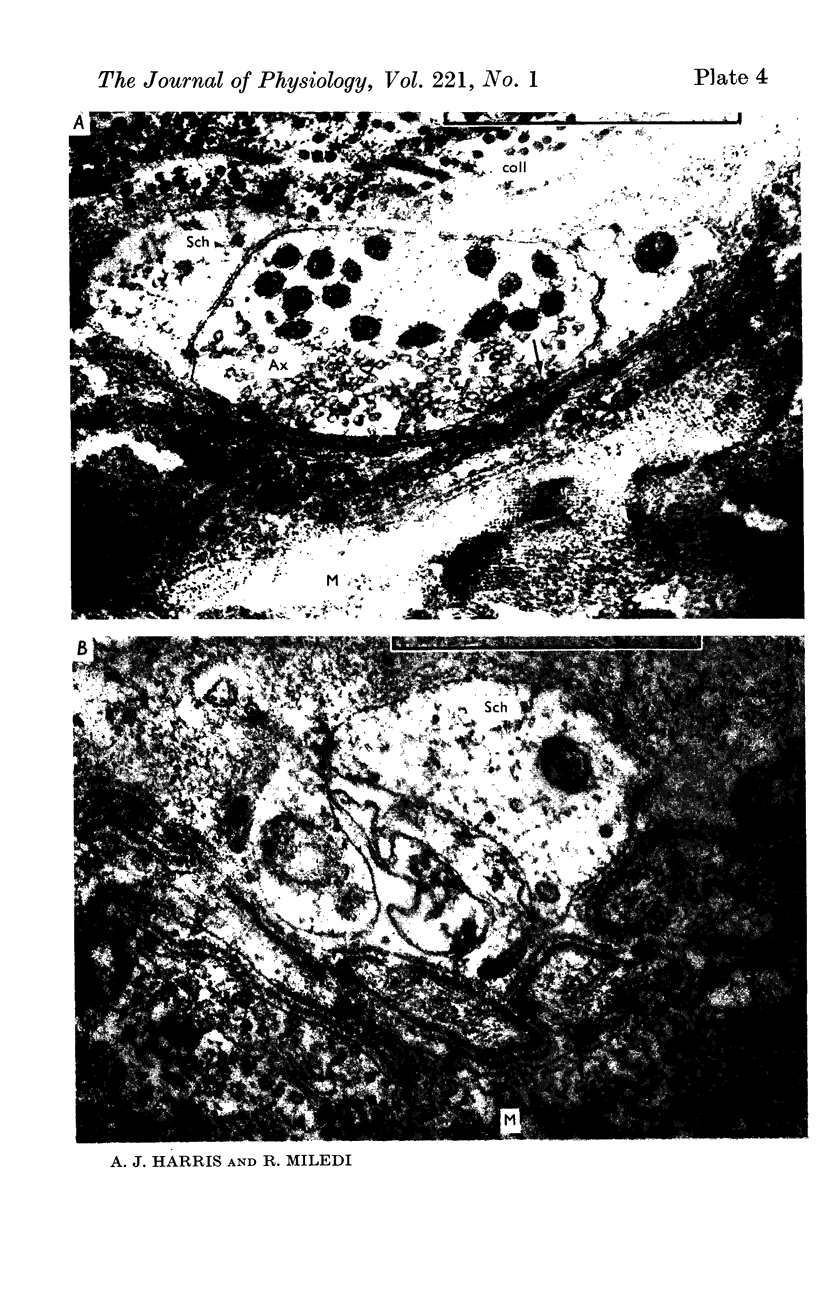
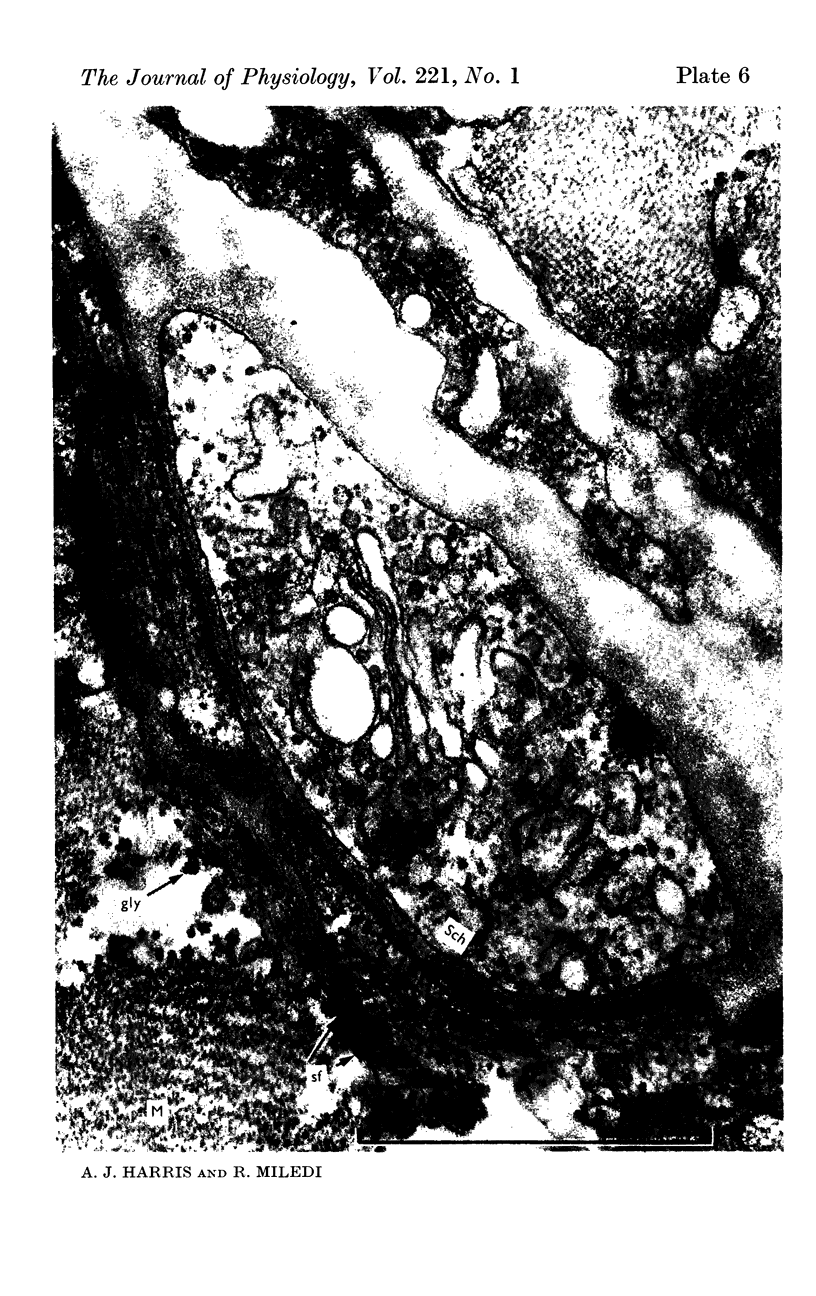
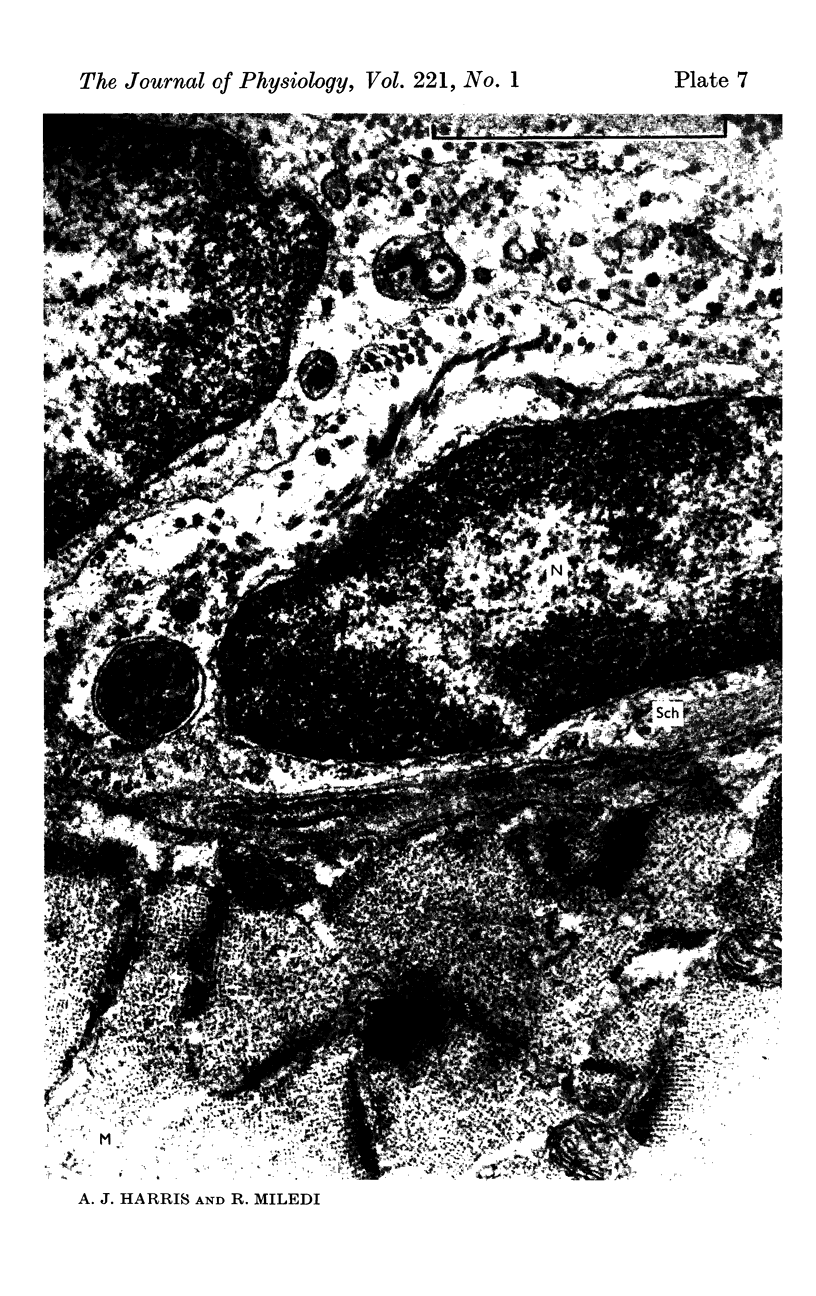
7. Electron microscopy shows that muscle structure is well preserved in culture, that the axons degenerate and that the Schwann cells move to occupy the space vacated by the axons. The Schwann cells are very probably the source of the acetylcholine which evokes miniature potentials in the denervated end-plates.
Full text
PDF




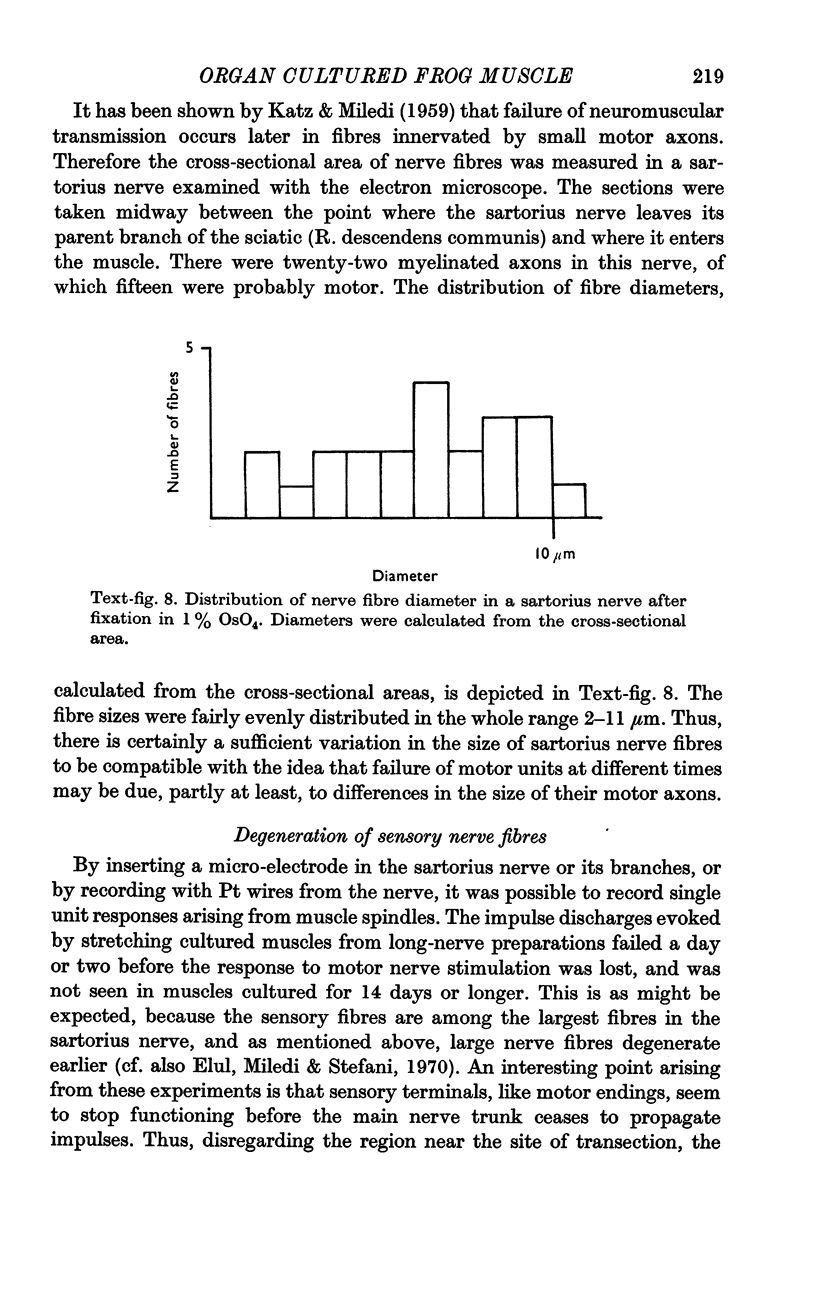






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushny A. R. On the exhalation of drugs by the lungs. J Physiol. 1910 Apr 26;40(1-2):17–27. doi: 10.1113/jphysiol.1910.sp001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul R., Miledi R., Stefani E. Neural control of contracture in slow muscle fibres of the frog. Acta Physiol Lat Am. 1970;20(3):194–226. [PubMed] [Google Scholar]
- Elul R., Miledi R., Stefani E. Neurotrophic control of contracture in slow muscle fibres. Nature. 1968 Mar 30;217(5135):1274–1275. doi: 10.1038/2171274a0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine sensitivity of muscle fiber membranes: mechanism of regulation by motoneurons. Science. 1970 Apr 17;168(3929):372–373. doi: 10.1126/science.168.3929.372. [DOI] [PubMed] [Google Scholar]
- Feng T. P. The effect of length on the resting metabolism of muscle. J Physiol. 1932 Apr 26;74(4):441–454. doi: 10.1113/jphysiol.1932.sp002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957 Sep 25;3(5):631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. Prolonged survival of isolated frog muscle and its sensitivity to acetylcholine. Nature. 1966 Feb 12;209(5024):716–717. doi: 10.1038/209716a0. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol. 1953 Aug;121(2):318–340. doi: 10.1113/jphysiol.1953.sp004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L. The effects of modified Ringer's solutions on the membrane potentials of innervated and denervated frog sartorius muscle fibers. J Cell Physiol. 1966 Feb;67(1):107–123. doi: 10.1002/jcp.1040670113. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R., TROWELL O. A. Acetylcholine sensitivity of rat diaphragm maintained in organ culture. Nature. 1962 Jun 9;194:981–982. doi: 10.1038/194981a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Stefani E. Miniature potentials in denervated slow muscle fibres of the frog. J Physiol. 1970 Jul;209(1):179–186. doi: 10.1113/jphysiol.1970.sp009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACHEY L. D., HUXLEY A. F. Structural identification of twitch and slow striated muscle fibers of the frog. J Cell Biol. 1962 Apr;13:177–180. doi: 10.1083/jcb.13.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Effects of motor innervation on the chemical sensitivity of skeletal muscle. Physiol Rev. 1960 Oct;40:734–752. doi: 10.1152/physrev.1960.40.4.734. [DOI] [PubMed] [Google Scholar]









