Abstract
1. Slices or bits of rabbit tissues, not exceeding 100 mg, were incubated in tissue culture medium containing tritium-labelled prostaglandin ([3H]PG). In some experiments, incubation medium also contained saturating concentrations of an unlabelled prostaglandin (PG), or [14C]-sucrose for determination of extracellular space. At the end of the incubation period, usually 1 hr, the tissues were removed and weighed, and their 3H (and 14C) content were determined along with that of a unit volume of medium.
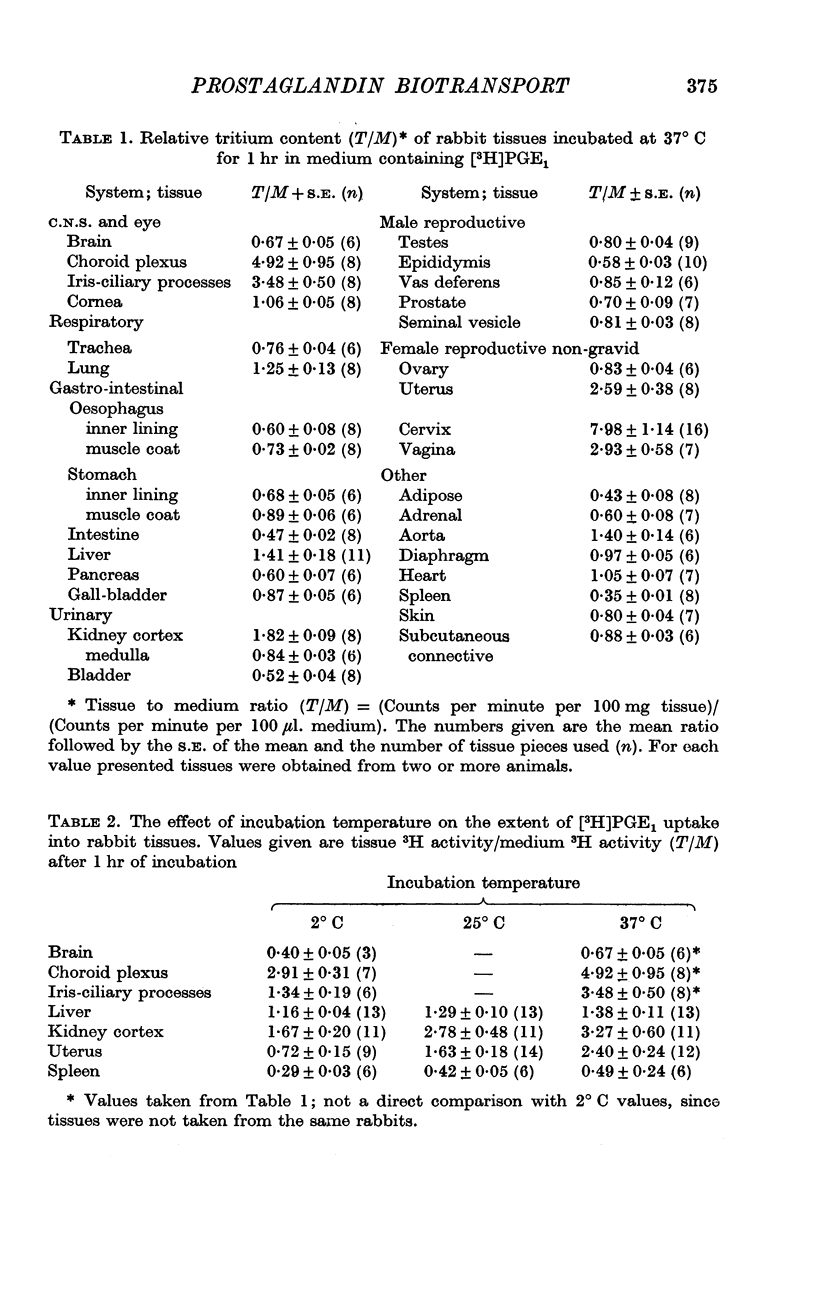
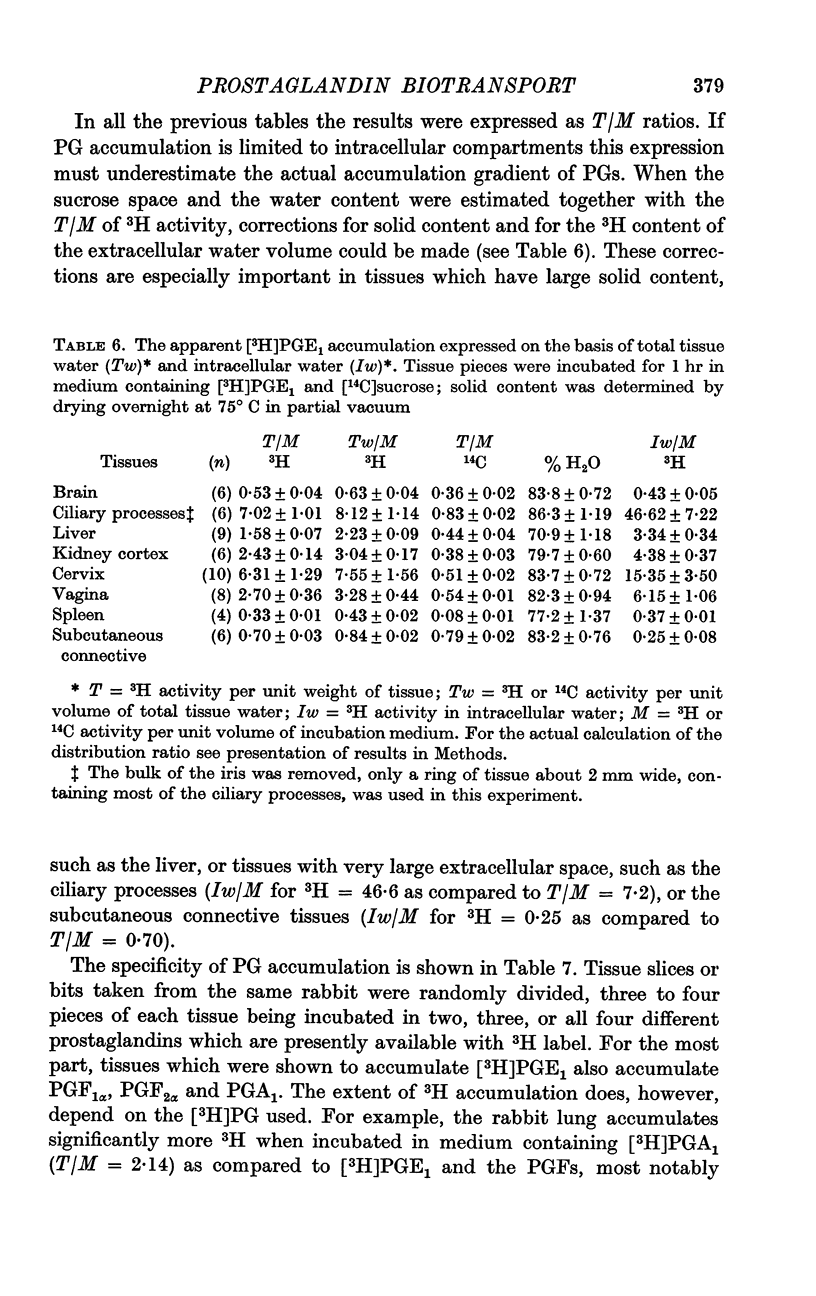
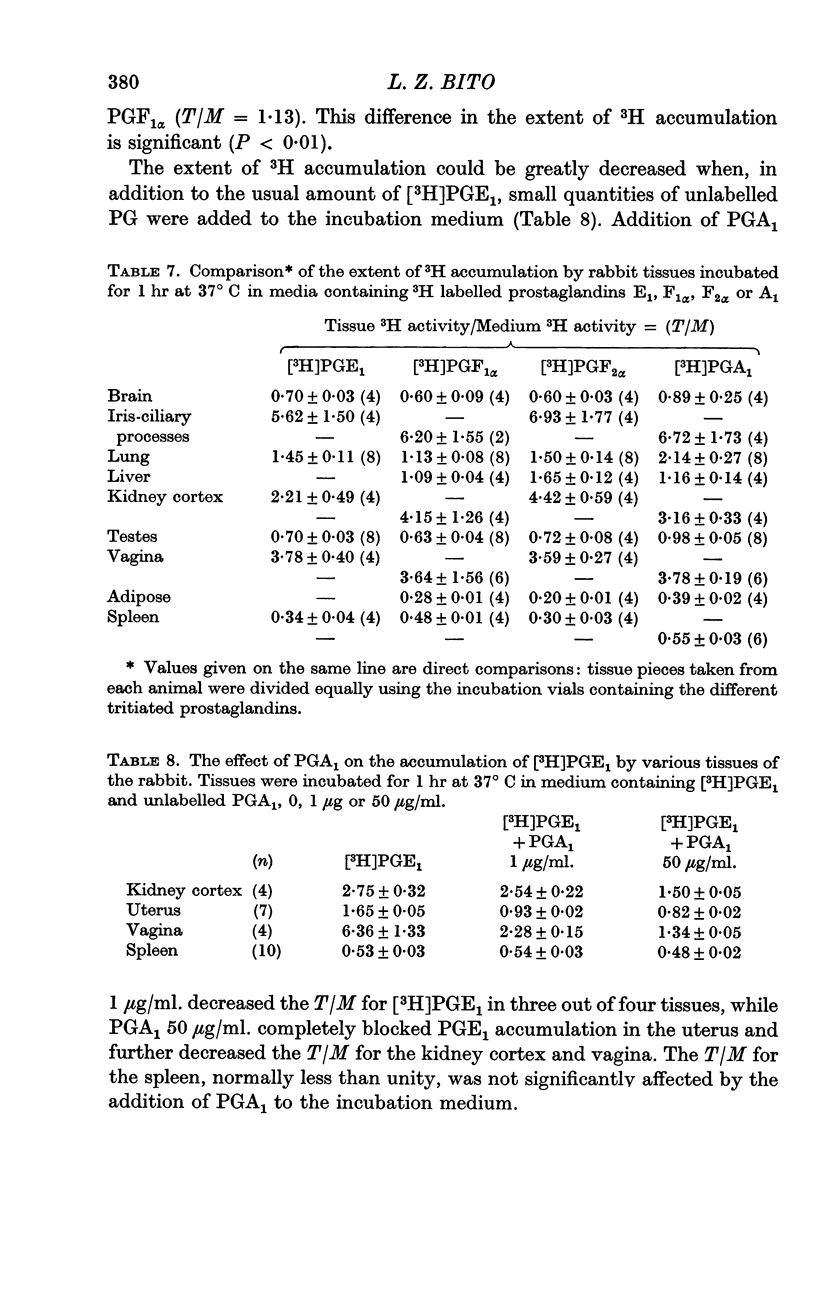
2. Tissues known to play a central role in PG metabolism (lung and liver) and in its excretion (kidney cortex) and tissues which have a known function in blood-brain and blood-ocular barriers (choroid plexuses and ciliary processes) show a large accumulation of 3H when incubated in a medium containing [3H]PGE1. In addition, tissues of the female reproductive tract, and the aorta of the rabbit show similar 3H accumulation. When uncorrected for tissue solid content or extracellular water volume, the extent of this accumulation is two- to sixfold. Calculated on the basis that all excess 3H is present in the free form in the intracellular water, the accumulation ratio for ciliary processes, for example, indicates an over fortysix-fold gradient of PGE1 across the cell membrane.
3. Tissues which accumulate [3H]PGE1 also accumulate [3H]PGA1, [3H]PGF1α and [3H]PGF2α. In some tissues specificity is, however, apparent; in the lung accumulation of [3H]PGA1 was significantly greater than that of [3H]PGF1α.
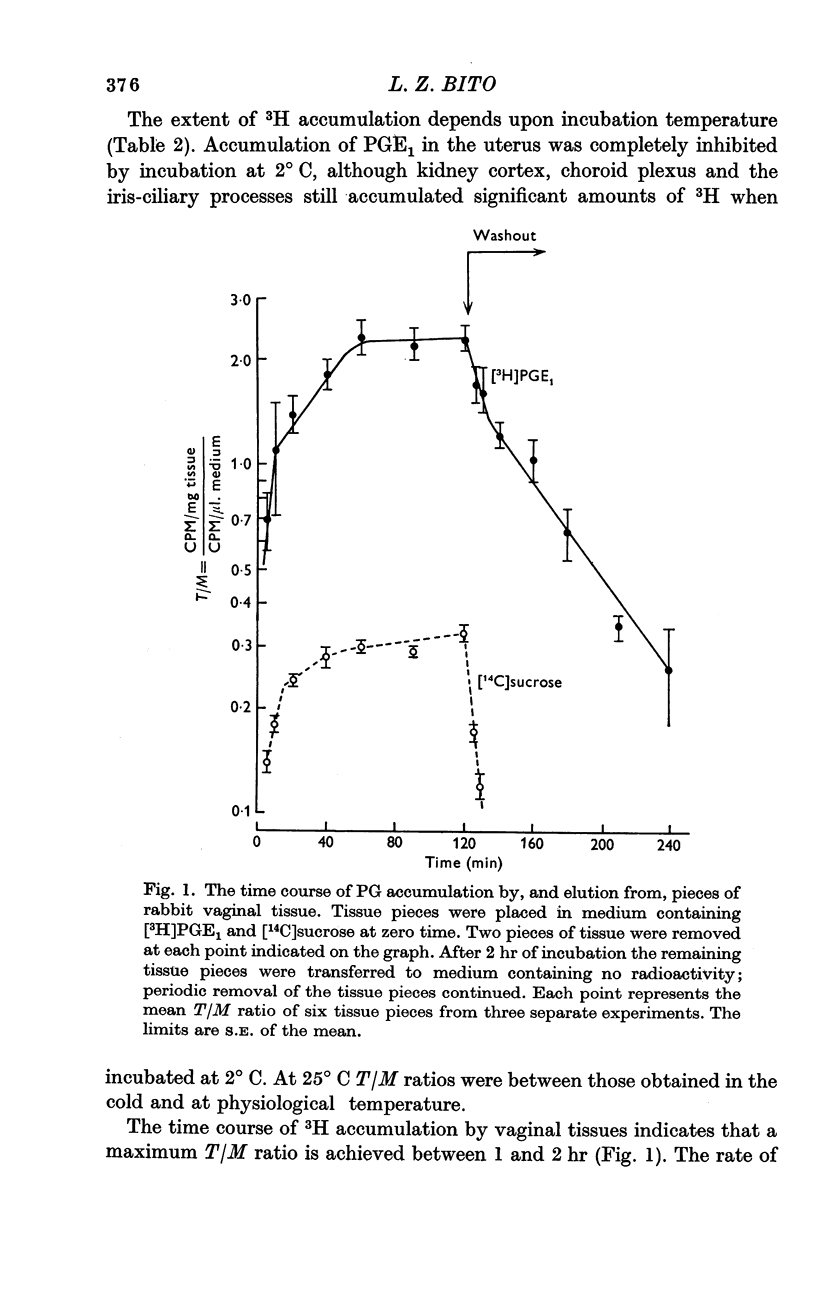
4. The extent of [3H]PGE1 accumulation was decreased, or in some tissues completely inhibited, by incubation at 2° C, or by addition of large concentrations of unlabelled PG.
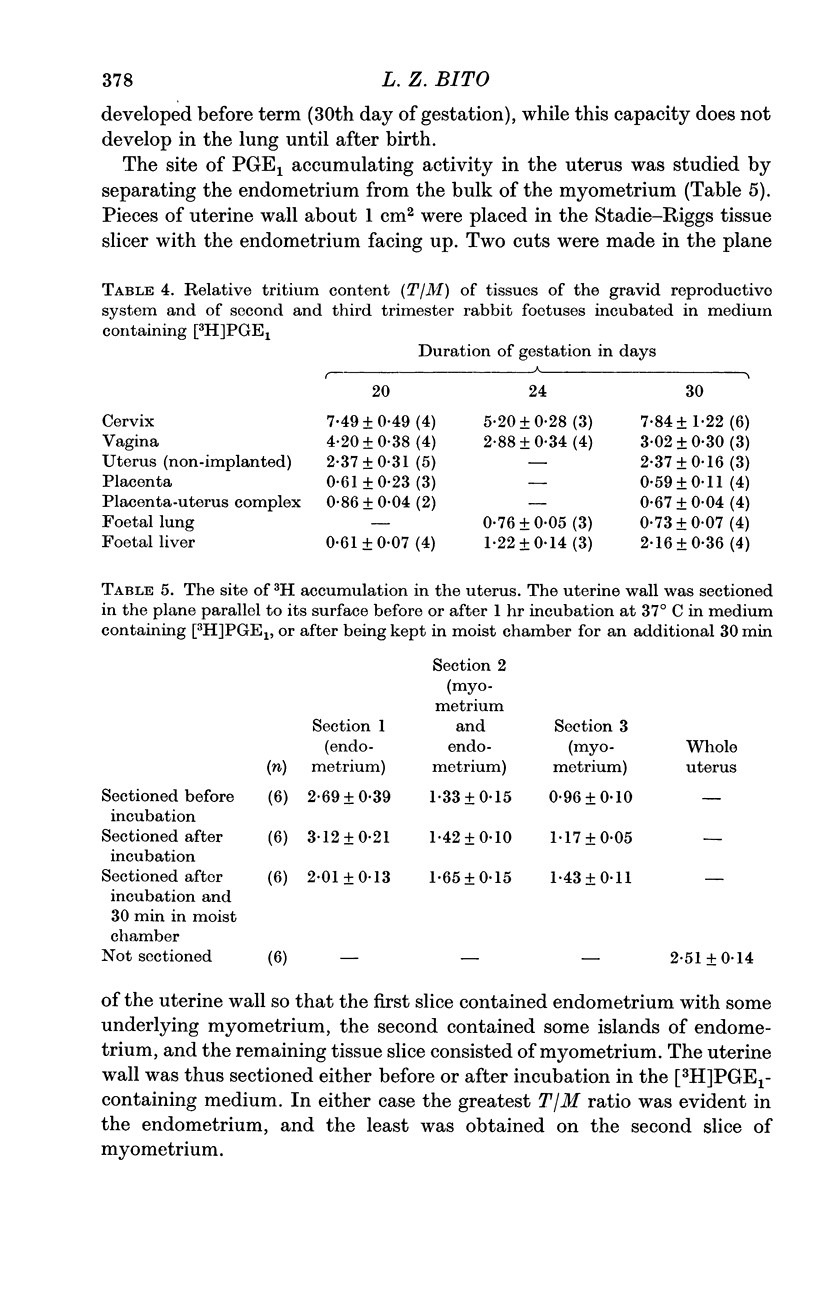
5. Accumulation of [3H]PGE1 by the foetal liver is not apparent on the 20th day of gestation, but is fully developed by the 30th day of gestation. The foetal lung does not accumulate [3H]PGE1 at any stage of gestation.
6. In some tissues, most notably muscle, there appears to be full equilibrium of [3H]PGE1 between tissue water and medium within 1 hr of incubation.
7. PGs are partially excluded from the intracellular volume of some other tissues, most notably the spleen and subcutaneous connective tissues. This apparent exclusion cannot be blocked by incubation in the cold, or by the addition of saturating levels of unlabelled PG.
8. The simplest explanation for all observed results is that cell membranes are, in general, impermeable to PGs. However, there are specific, carrier-mediated mechanisms across some membranes which facilitate the entry of PGs. In some cells these transport mechanisms are linked to a source of metabolic energy, and/or to the counter-transport of some other substance, thus allowing net accumulation of PGs against a concentration gradient. Alternatively, 3H accumulation may represent adsorption of [3H]PGs or one of their labelled metabolites on to specific adsorption sites.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N., KAVANAGH L., WHITING J. EFFECT OF MECHANICAL STIMULATION ON RABBITS' EYES: RELEASE OF ACTIVE SUBSTANCE IN ANTERIOR CHAMBER PERFUSATES. J Physiol. 1965 Feb;176:378–408. doi: 10.1113/jphysiol.1965.sp007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER B. Cerebrospinal fluid iodide. Am J Physiol. 1961 Dec;201:1149–1151. doi: 10.1152/ajplegacy.1961.201.6.1149. [DOI] [PubMed] [Google Scholar]
- BECKER B. The transport of organic anions by the rabbit eye. I. In vitro iodopyracet (Diodrast) accumulation by ciliary body-iris preparations. Am J Ophthalmol. 1960 Nov;50:862–867. doi: 10.1016/0002-9394(60)90338-x. [DOI] [PubMed] [Google Scholar]
- Barrett S., Blockley M. A., Brown J. M., Cumming I. A., Goding J. R., Mole B. J., Obst J. M. Initiation of the oestrous cycle in the ewe by infusions of PGF 2 alpha-to the autotransplanted ovary. J Reprod Fertil. 1971 Jan;24(1):136–137. doi: 10.1530/jrf.0.0240136. [DOI] [PubMed] [Google Scholar]
- CROSS R. J., TAGGART J. V. Renal tubular transport: accumulation of p-aminohippurate by rabbit kidney slices. Am J Physiol. 1950 Apr 1;161(1):181–190. doi: 10.1152/ajplegacy.1950.161.1.181. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Rigor B. M. The choroid plexus as a glucose barrier. Prog Brain Res. 1968;29:147–158. doi: 10.1016/S0079-6123(08)64153-9. [DOI] [PubMed] [Google Scholar]
- Dawson W., Jessup S. J., McDonald-Gibson W., Ramwell P. W., Shaw J. E. Prostaglandin uptake and metabolism by the perfused rat liver. Br J Pharmacol. 1970 Jul;39(3):585–598. doi: 10.1111/j.1476-5381.1970.tb10366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTON E. W. ACTIONS OF PROSTAGLANDINS E1, E2 AND E3 ON THE CENTRAL NERVOUS SYSTEM. Br J Pharmacol Chemother. 1964 Feb;22:189–192. doi: 10.1111/j.1476-5381.1964.tb01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. Metabolism of prostaglandins in rat liver mitochondria. Eur J Biochem. 1968 Oct 17;6(1):135–146. doi: 10.1111/j.1432-1033.1968.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Hansson E., Samuelsson B. Autoradiographic distribution studies of 3H-labeled prostaglandin E1 in mice. Prostaglandins and related factors 31. Biochim Biophys Acta. 1965 Oct 4;106(2):379–385. doi: 10.1016/0005-2760(65)90046-9. [DOI] [PubMed] [Google Scholar]
- Holmes S. W. The spontaneous release of prostaglandins into the cerebral ventricles of the dog and the effect of external factors on this release. Br J Pharmacol. 1970 Apr;38(4):653–658. doi: 10.1111/j.1476-5381.1970.tb09874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton E. W., Main I. H. Further observations on the central nervous actions of prostaglandins F2a and E1. With an addendum on the effects of prostglandins E1 and F2a on systemic arterial blood pressure in chicks. Br J Pharmacol Chemother. 1967 Aug;30(3):568–581. doi: 10.1111/j.1476-5381.1967.tb02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S. M. Identification of prostaglandins in human amniotic fluid. J Obstet Gynaecol Br Commonw. 1966 Dec;73(6):903–908. doi: 10.1111/j.1471-0528.1966.tb06112.x. [DOI] [PubMed] [Google Scholar]
- McCracken J. Prostaglandin F-2 alpha and corpus luteum regression. Ann N Y Acad Sci. 1971 Apr 30;180:456–472. doi: 10.1111/j.1749-6632.1971.tb53213.x. [DOI] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Spontaneous and evoked release of prostaglandins from cerebral cortex of anesthetized cats. Am J Physiol. 1966 Jul;211(1):125–134. doi: 10.1152/ajplegacy.1966.211.1.125. [DOI] [PubMed] [Google Scholar]
- Sandberg F., Ingelman-Sundberg A., Rydén G., Joelsson I. The absorption of tritium-labelled prostaglandin E1 from the vagina of non-pregnant women. Acta Obstet Gynecol Scand. 1968;47(1):22–26. doi: 10.3109/00016346809157462. [DOI] [PubMed] [Google Scholar]


