Abstract
1. Spike discharges were recorded from neurones in the lumbar spinal cord in cats anaesthetized by barbiturate.
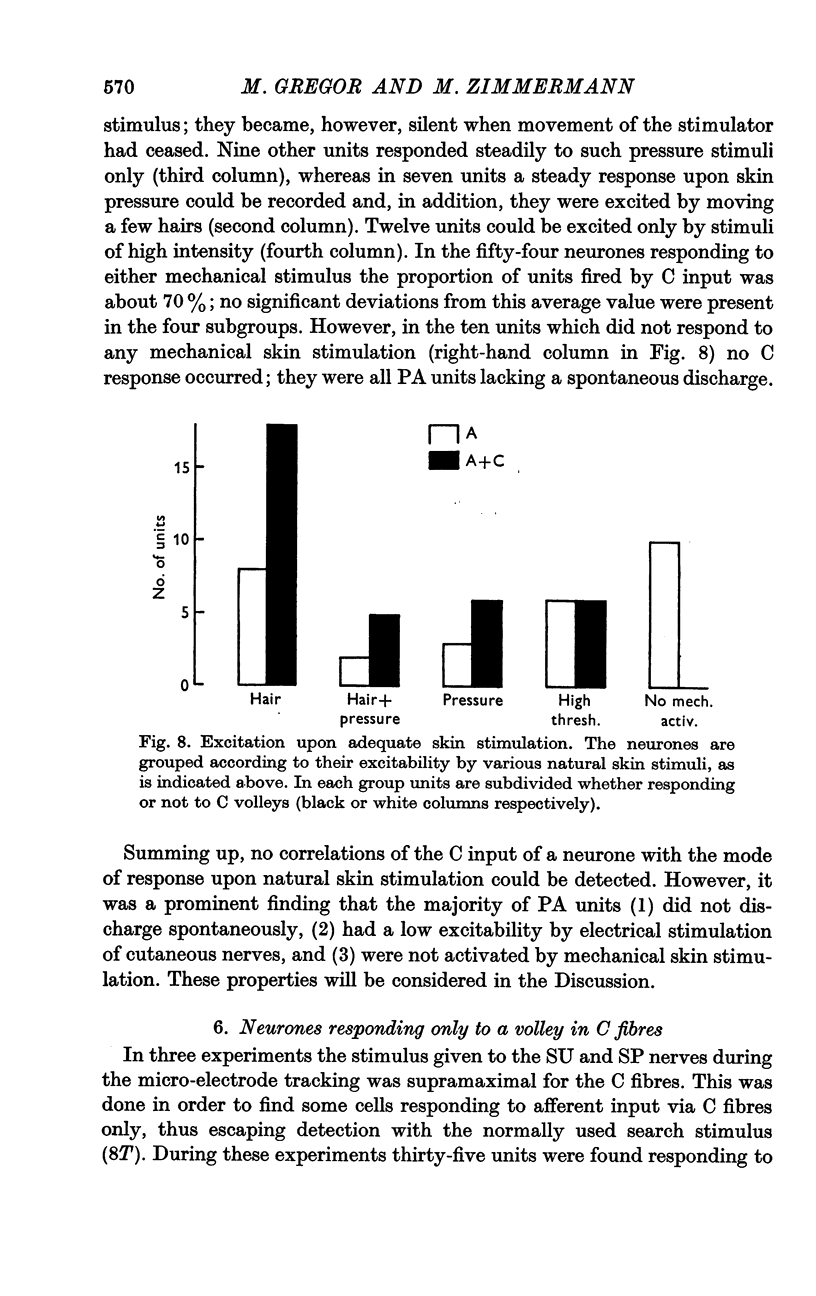
2. The neurones were examined systematically for various physiological parameters and for their location. Especially the neurones situated in the dorsal horn were classified for the following parameters: mono- or polysynaptic linkage to myelinated afferents; type of natural stimuli which excited the neurones; depth from the cord surface; number of impulses discharged upon a cutaneous A fibre stimulus; steady-state discharge in the absence of intentional stimulation.
3. All neurones were also tested as to whether or not they responded to volleys in cutaneous C fibres. Of 111 units which were activated by the A fibres in nerves from the hairy skin, 57 (= 51%) responded to C volleys in those nerves too.
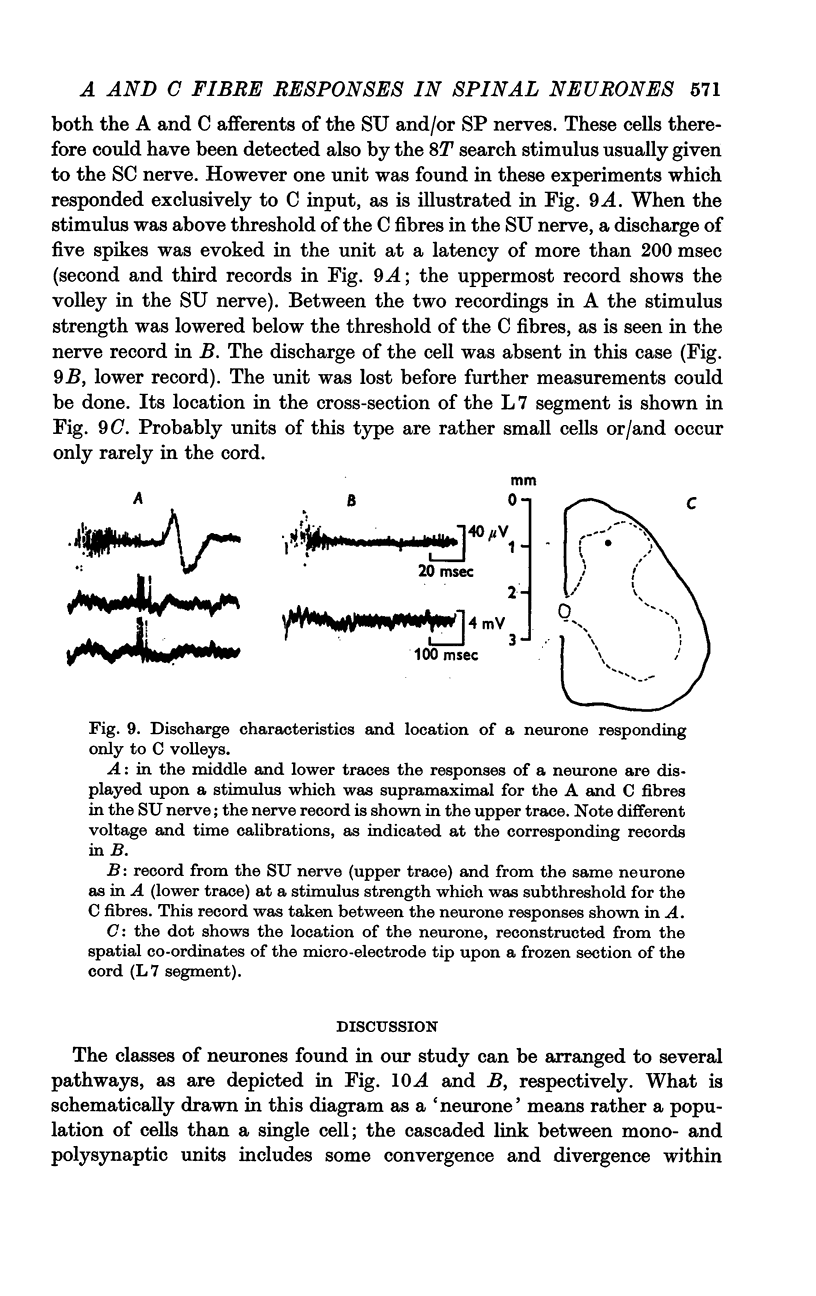
4. By blocking conduction in the A fibres using polarizing currents it was shown that the responses to C fibre volleys were partially or totally suppressed by a preceding discharge of the neurone in response to an A volley. Using search stimuli which were suprathreshold for C fibres one cell out of 36 could be found which responded only to afferent volleys in C fibres.
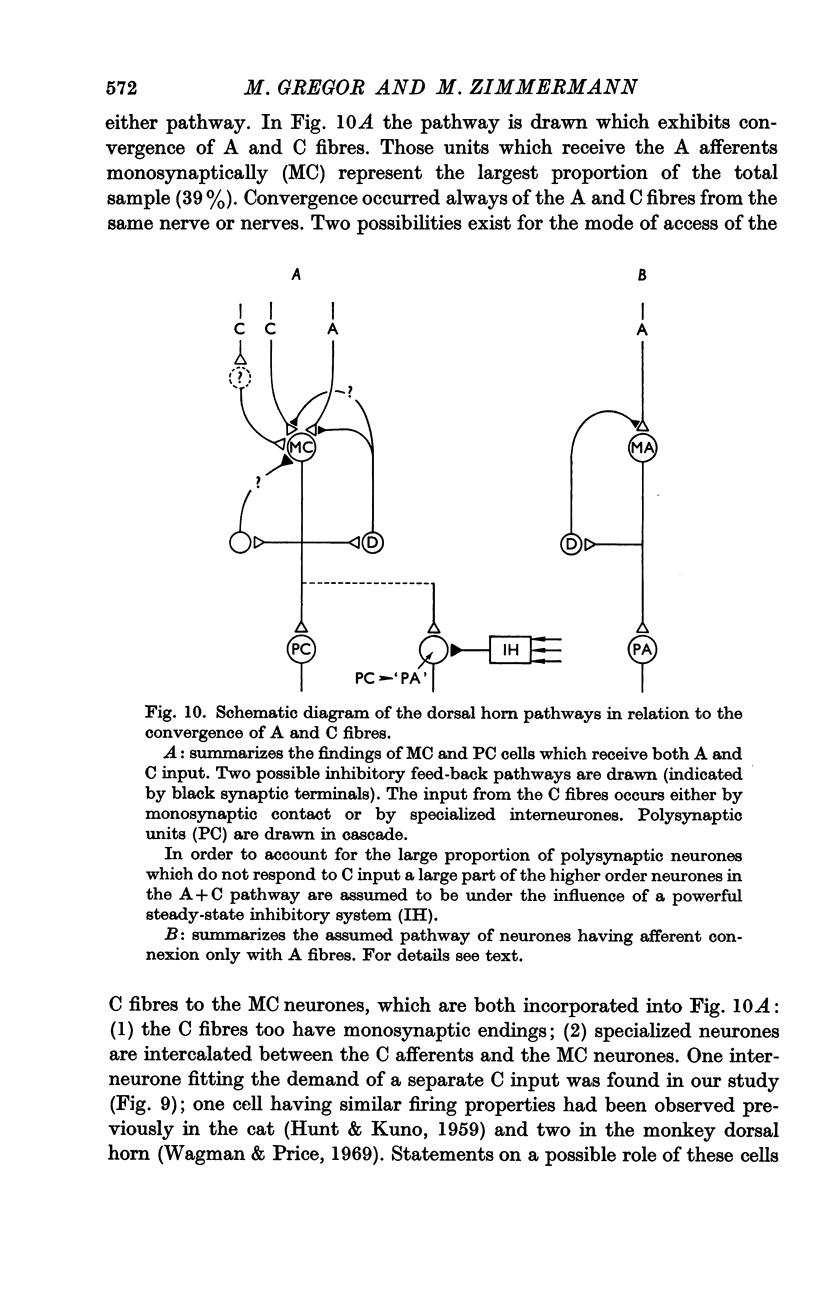
5. About half of all neurones were shown to be connected monosynaptically to cutaneous A fibres, as was judged from the synaptic delay. The other half were polysynaptically linked to the A fibres. Both mono- and polysynaptic neurones were found in all layers of the dorsal horn. About 15% of the cells had additional input from muscle Group II and/or III fibres via polysynaptic pathways.
6. Subdividing the A and A+C responsive neurones according to their mono- (M) or polysynaptic (P) connexions yielded the following sub-samples: MC, 39%; PC, 15%; MA, 13%; PA, 33%. Most MC neurones had, and most PA units had not, a spontaneous discharge. About half of the PA cells could not be driven by natural skin stimulation. The majority of MC units responded specifically to movement of hairs.
7. A model was proposed hypothesizing two pathways in the dorsal horn, one showing convergence of A and C fibres and the other not. Some relations concerning other observations on C fibre effects were discussed.
Full text
PDF




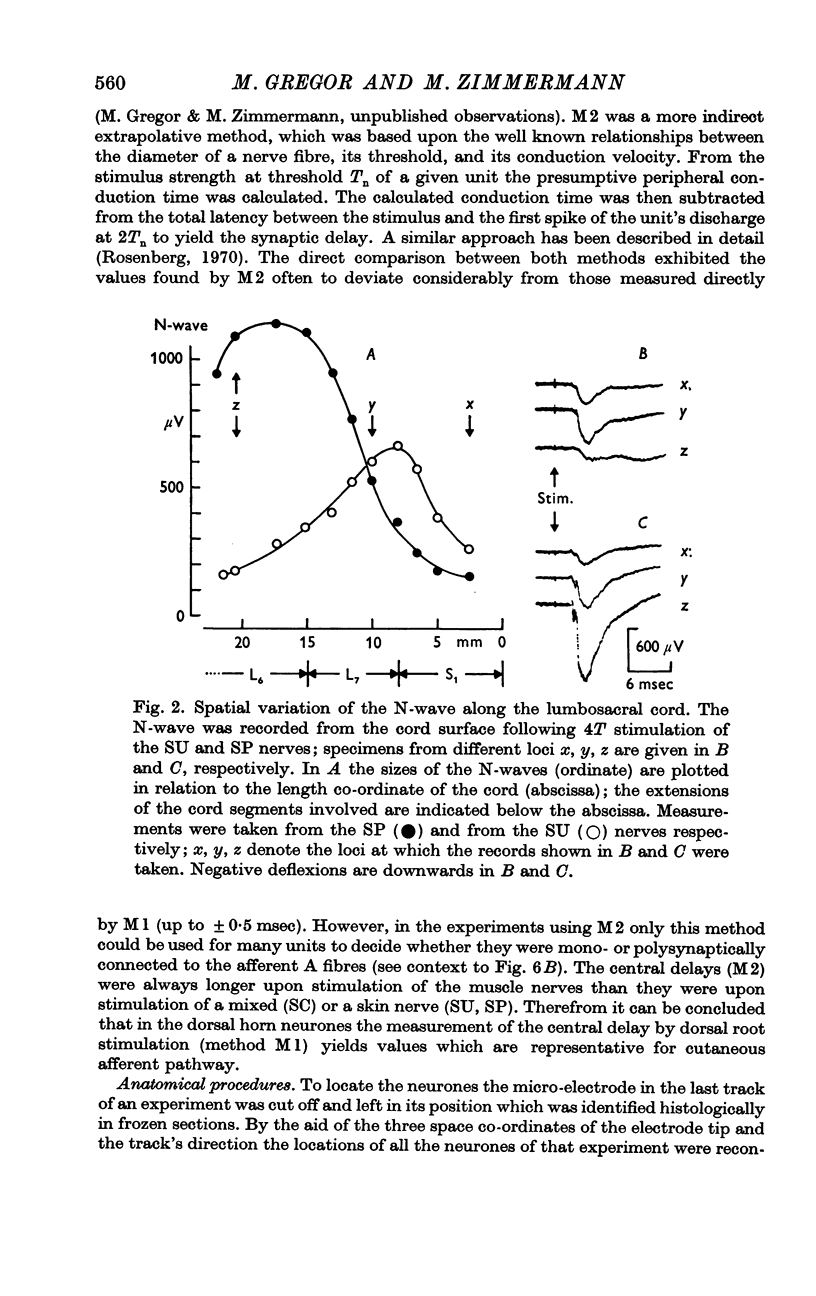


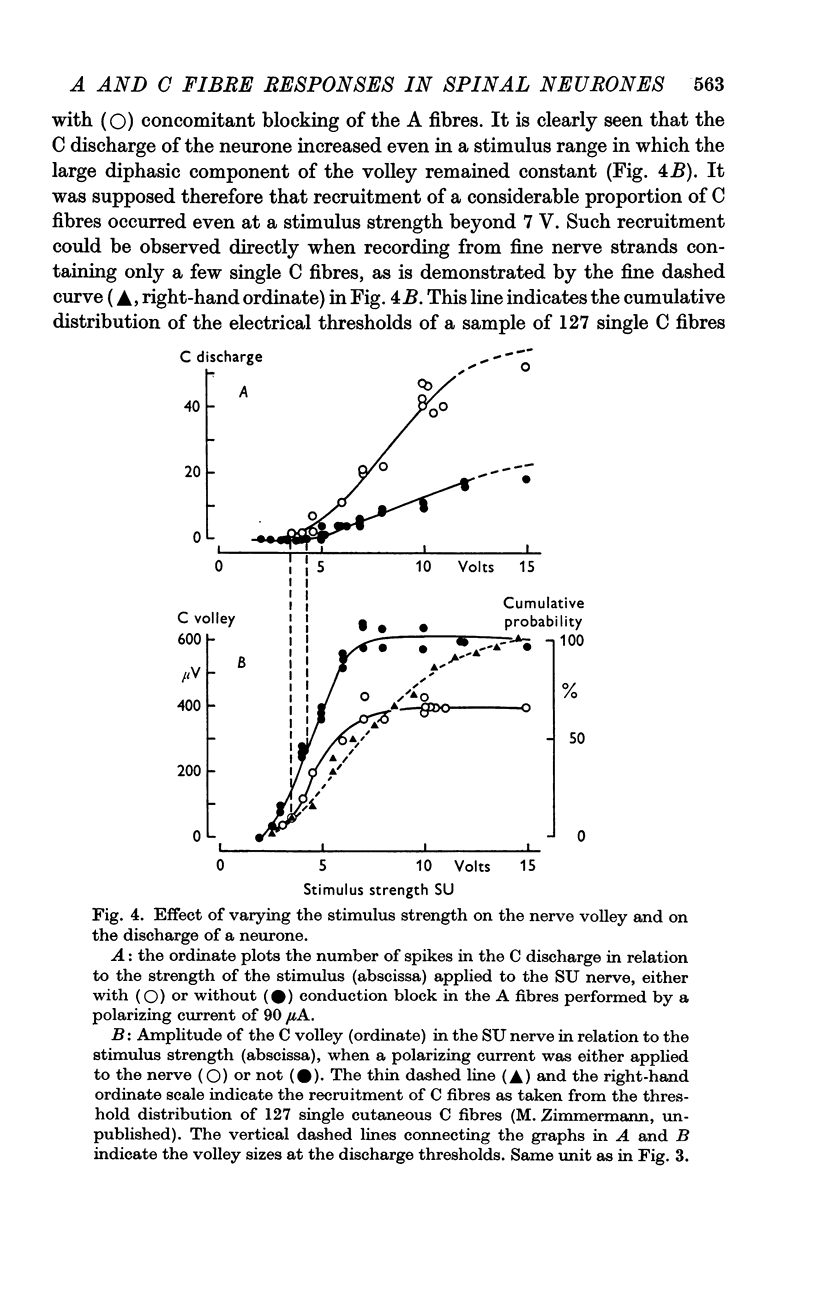


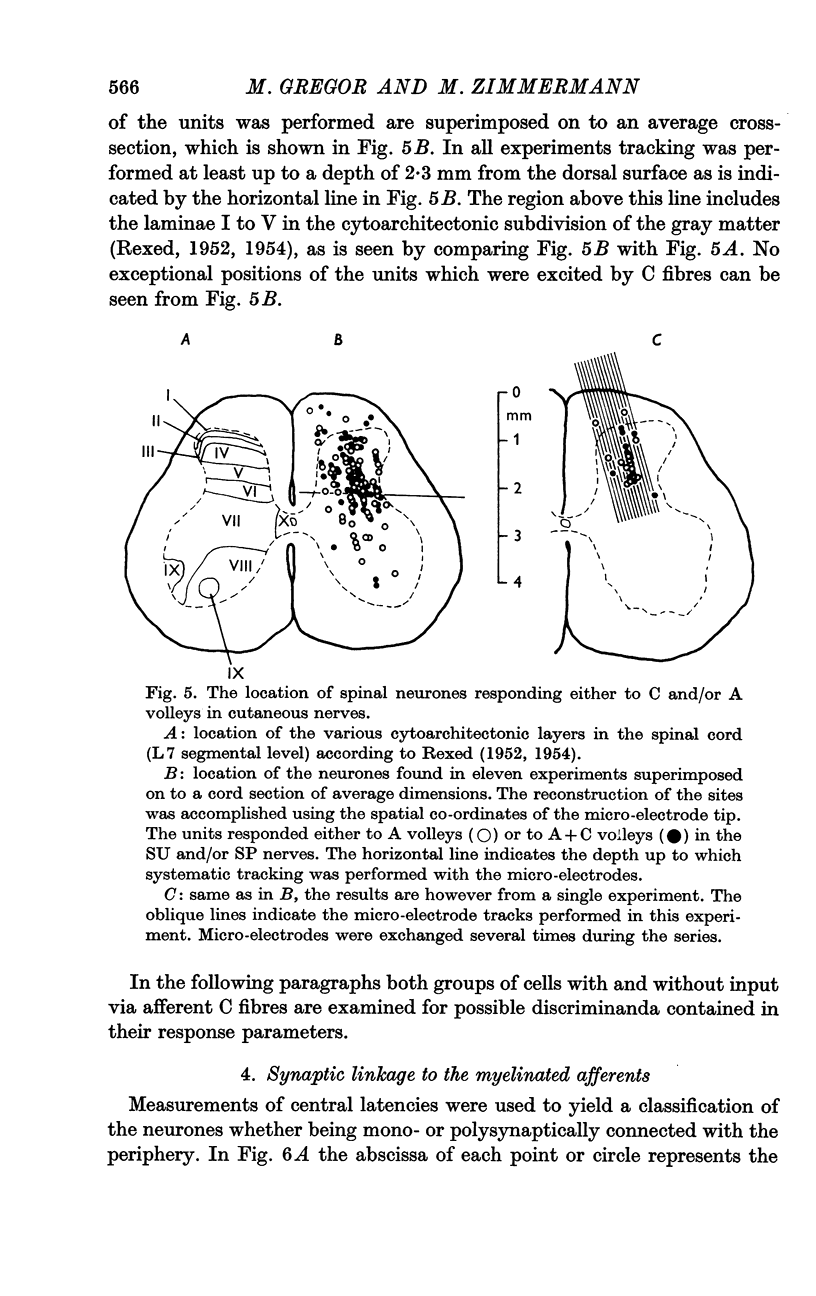

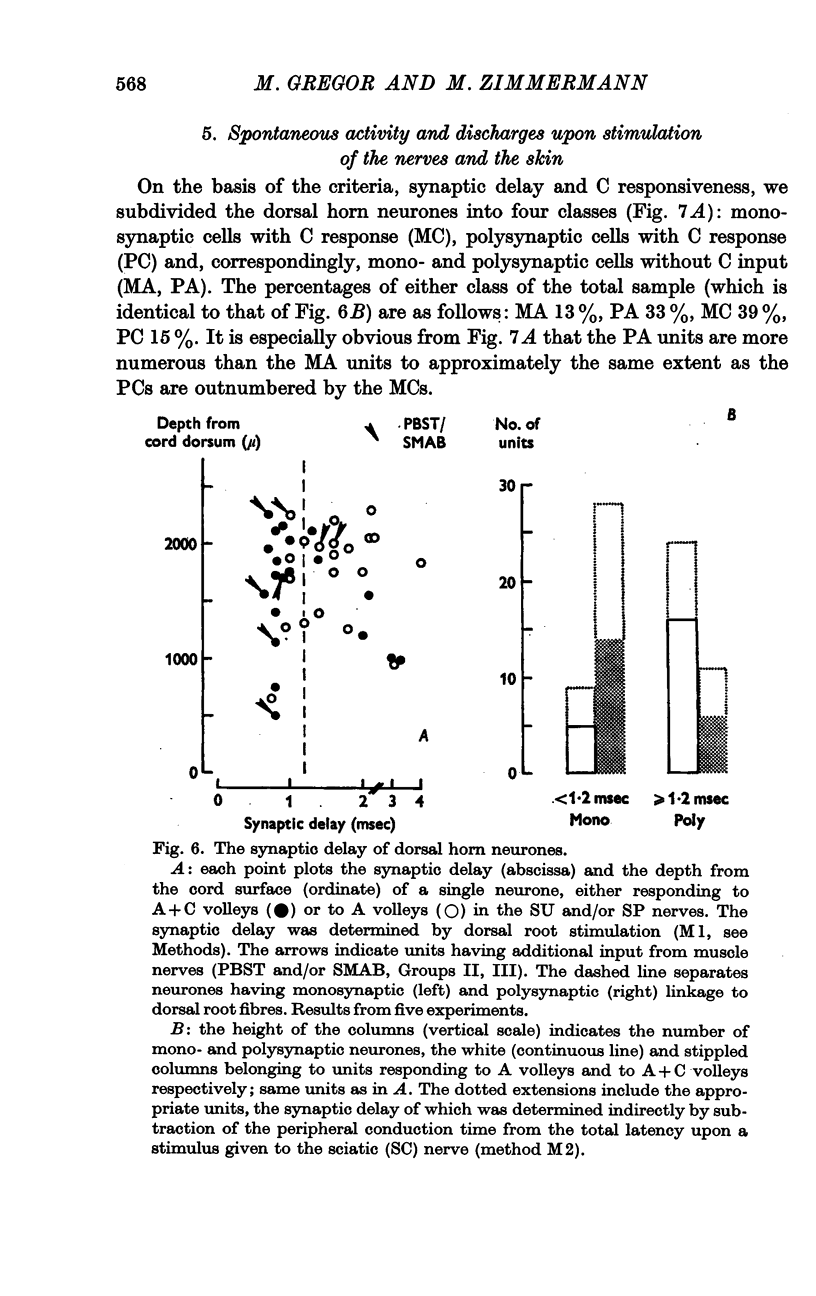





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., GRAY J. A., PALMER J. F. A group of neurones in the dorsal horn associated with cutaneous mechanoreceptors. J Physiol. 1961 May;156:611–622. doi: 10.1113/jphysiol.1961.sp006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. Nonmedullated fibres in the saphenous nerve which signal touch. J Physiol. 1957 Dec 31;139(3):385–399. doi: 10.1113/jphysiol.1957.sp005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M., STRAUB R. W. The role of nonmyelinated fibres in signalling cooling of the skin. J Physiol. 1960 Feb;150:266–283. doi: 10.1113/jphysiol.1960.sp006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Central pathways responsible for depolarization of primary afferent fibres. J Physiol. 1962 May;161:237–257. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Presynaptic inhibition of the central actions of flexor reflex afferents. J Physiol. 1962 May;161:258–281. doi: 10.1113/jphysiol.1962.sp006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E. Pyramidal tract effects on interneurons in the cat lumbar dorsal horn. J Neurophysiol. 1968 Jan;31(1):69–80. doi: 10.1152/jn.1968.31.1.69. [DOI] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Dorsal root potentials and ventral root reflexes evoked by nonmyelinated fibers. Science. 1968 Dec 6;162(3858):1140–1142. doi: 10.1126/science.162.3858.1140. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., KUNO M. Background discharge and evoked responses of spinal interneurones. J Physiol. 1959 Sep 2;147:364–384. doi: 10.1113/jphysiol.1959.sp006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRIUCHIJIMA J., ZOTTERMAN Y. The specificity of afferent cutaneous C fibres in mammals. Acta Physiol Scand. 1960 Jul 15;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- Jänig W., Zimmermann M. Presynaptic depolarization of myelinated afferent fibres evoked by stimulation of cutaneous C fibres. J Physiol. 1971 Apr;214(1):29–50. doi: 10.1113/jphysiol.1971.sp009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., MONTASTRUC P. Role des différents types de fibres afférentes dans les réflexes circulatoires généraux d'origine cutanée. J Physiol (Paris) 1957 Nov;49(5):1039–1049. [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. PRESYNAPTIC HYPERPOLARIZATION: A ROLE FOR FINE AFFERENT FIBRES. J Physiol. 1964 Aug;172:274–294. doi: 10.1113/jphysiol.1964.sp007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. RESPONSES OF SINGLE DORSAL CORD CELLS TO PERIPHERAL CUTANEOUS UNMYELINATED FIBRES. Nature. 1965 Apr 3;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Manfredi M., Castellucci V. C-fiber responses in the ventrolateral column of the cat spinal cord. Science. 1969 Sep 5;165(3897):1020–1022. doi: 10.1126/science.165.3897.1020. [DOI] [PubMed] [Google Scholar]
- Manfredi M. Modulation of sensory projections in anterolateral column of cat spinal cord by peripheral afferents of different size. Arch Ital Biol. 1970 Jan;108(1):72–105. [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E. Synaptic connexions of alpha extensor motoneurones with ipsilateral and contralateral cutaneous nerves. J Physiol. 1970 Mar;207(1):231–255. doi: 10.1113/jphysiol.1970.sp009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. NEURONAL AND SYNAPTIC ARRANGEMENT IN THE SUBSTANTIA GELATINOSA ROLANDI. J Comp Neurol. 1964 Apr;122:219–239. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal patterns in cat spinal cord. 3. Primary afferent collaterals. Brain Res. 1969 May;13(3):417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Weller E. Reflex activity in the cervical and lumbar sympathetic trunk induced by unmyelinated somatic afferents. Brain Res. 1970 Dec 1;24(2):207–218. doi: 10.1016/0006-8993(70)90101-0. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Cord cells responding to touch, damage, and temperature of skin. J Neurophysiol. 1960 Mar;23:197–210. doi: 10.1152/jn.1960.23.2.197. [DOI] [PubMed] [Google Scholar]
- Wagman I. H., Price D. D. Responses of dorsal horn cells of M. mulatta to cutaneous and sural nerve A and C fiber stimuli. J Neurophysiol. 1969 Nov;32(6):803–817. doi: 10.1152/jn.1969.32.6.803. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Dorsal root potentials after C-fiber stimulation. Science. 1968 May 24;160(3830):896–898. doi: 10.1126/science.160.3830.896. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Selective activation of C-fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(4):329–333. doi: 10.1007/BF00362643. [DOI] [PubMed] [Google Scholar]


