Abstract
1. The pial surface of different regions of the central nervous system of the rabbit have been bathed with artificial cerebrospinal fluid (c.s.f.), containing different concentrations of potassium. The object has been to change the composition of the interstitial fluid with respect to this ion, where it is adjacent to subarachnoid c.s.f.
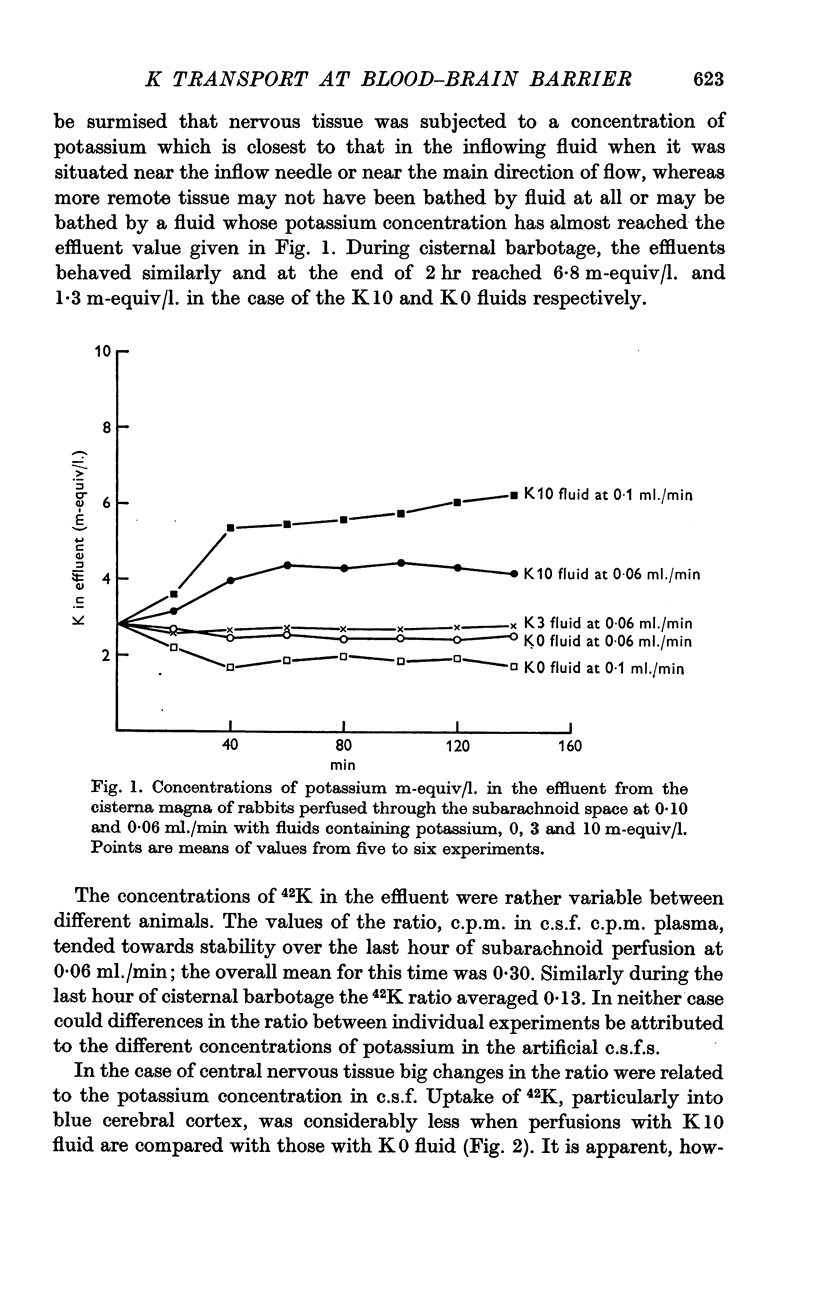
2. Two techniques, subarachnoid perfusion from the supracallosal space between the hemispheres to the cisterna magna and barbotage from the cisterna magna, have been used. If the artificial c.s.f. contains Evans Blue, the former procedure results in maximum staining of the pia and underlying nervous tissue of the pons-medulla and spinal cord. The latter procedure results in maximum staining of the medial and supero-lateral surfaces of the cerebral hemispheres, particularly anteriorly.
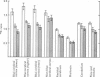
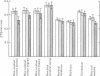
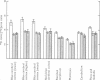
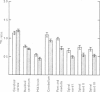
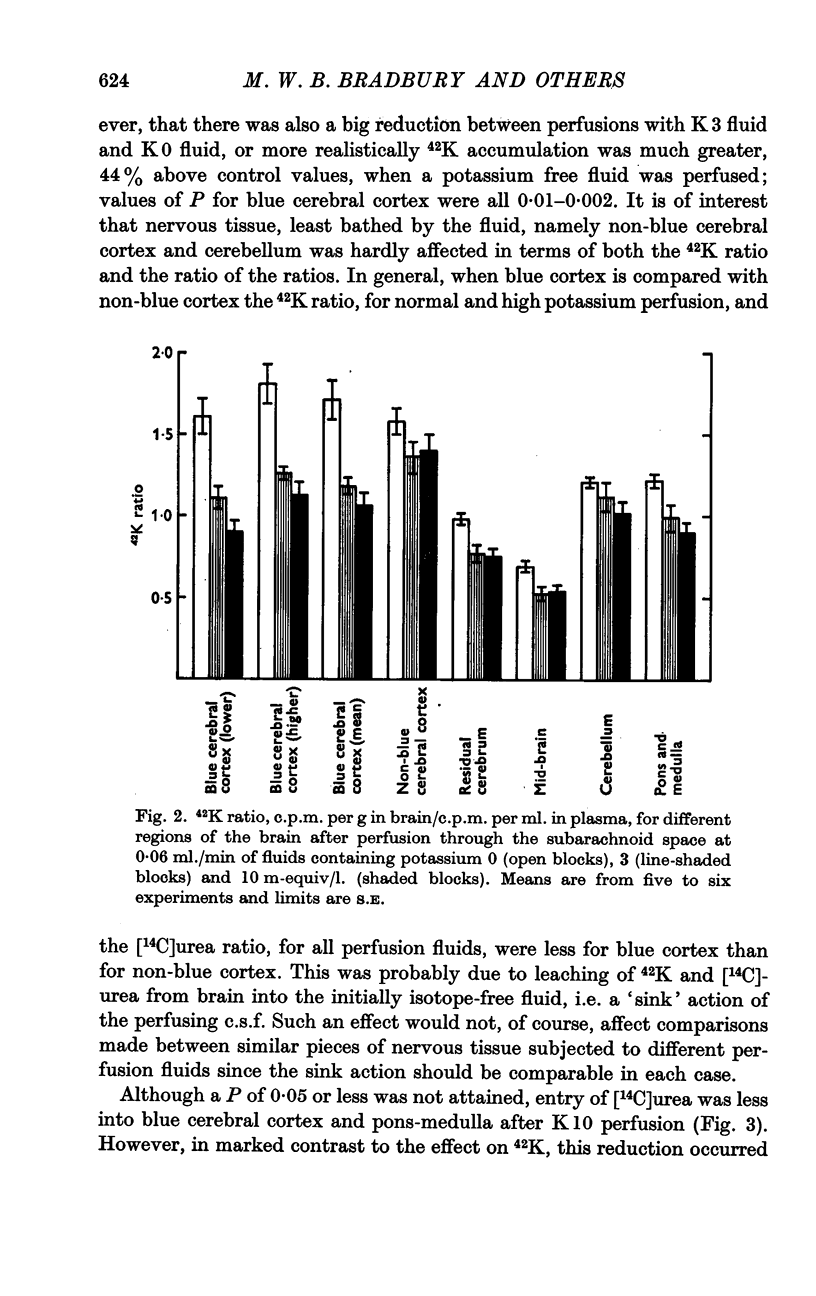
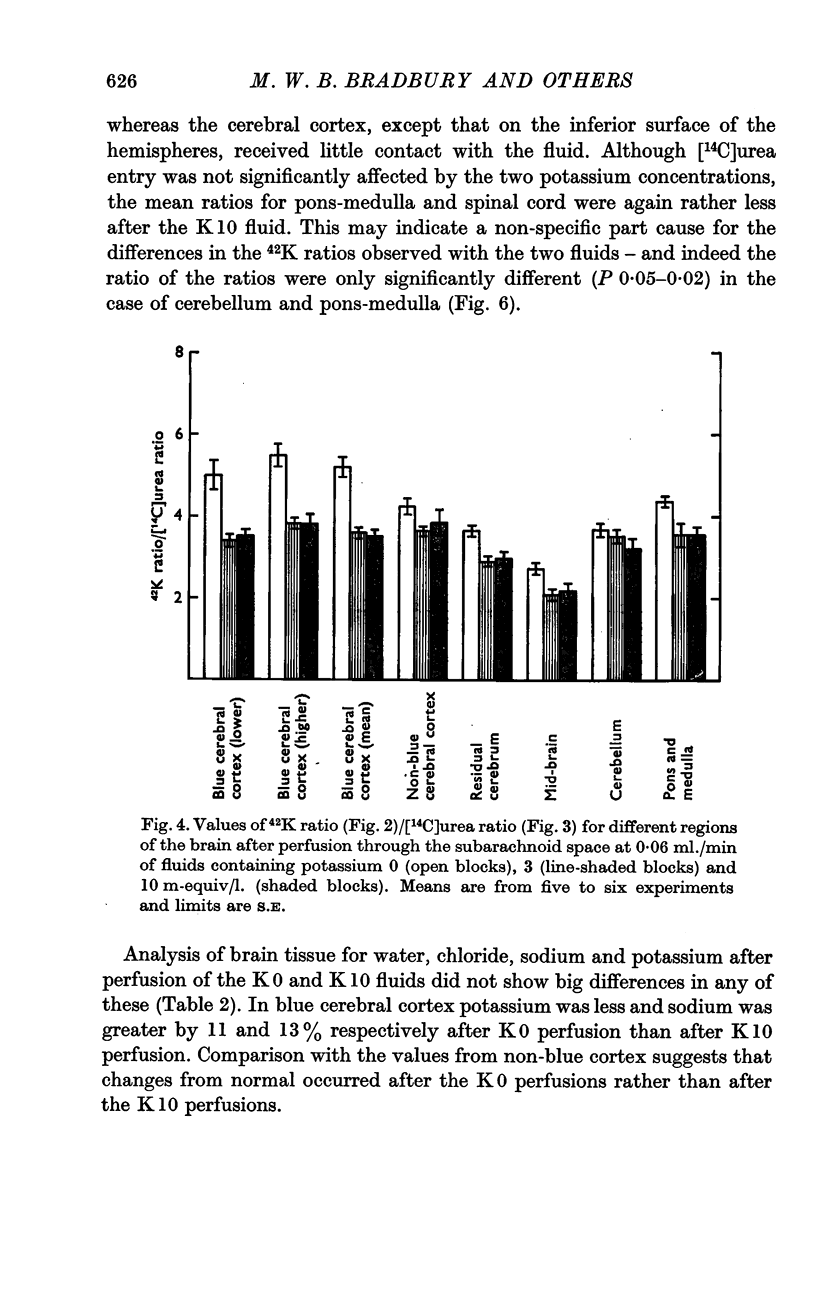
3. During subarachnoid perfusion at 0·06 ml./min with the potassium-free fluid, most regions of the brain took up significantly greater amounts of 42K than was the case during perfusion with the fluids containing 3 and 10 m-equiv/l. For blue cerebral cortex, the tissue subjected directly to the inflowing fluid and showing the biggest differences, the ratios, c.p.m. per g brain/c.p.m. per ml. plasma, were 1·71 ± 0·12 (+44%), 1·19 ± 0·05 and 1·07 ± 0·08 (-10%) during perfusion with the fluids containing, 0, 3 and 10 m-equiv/l. respectively.
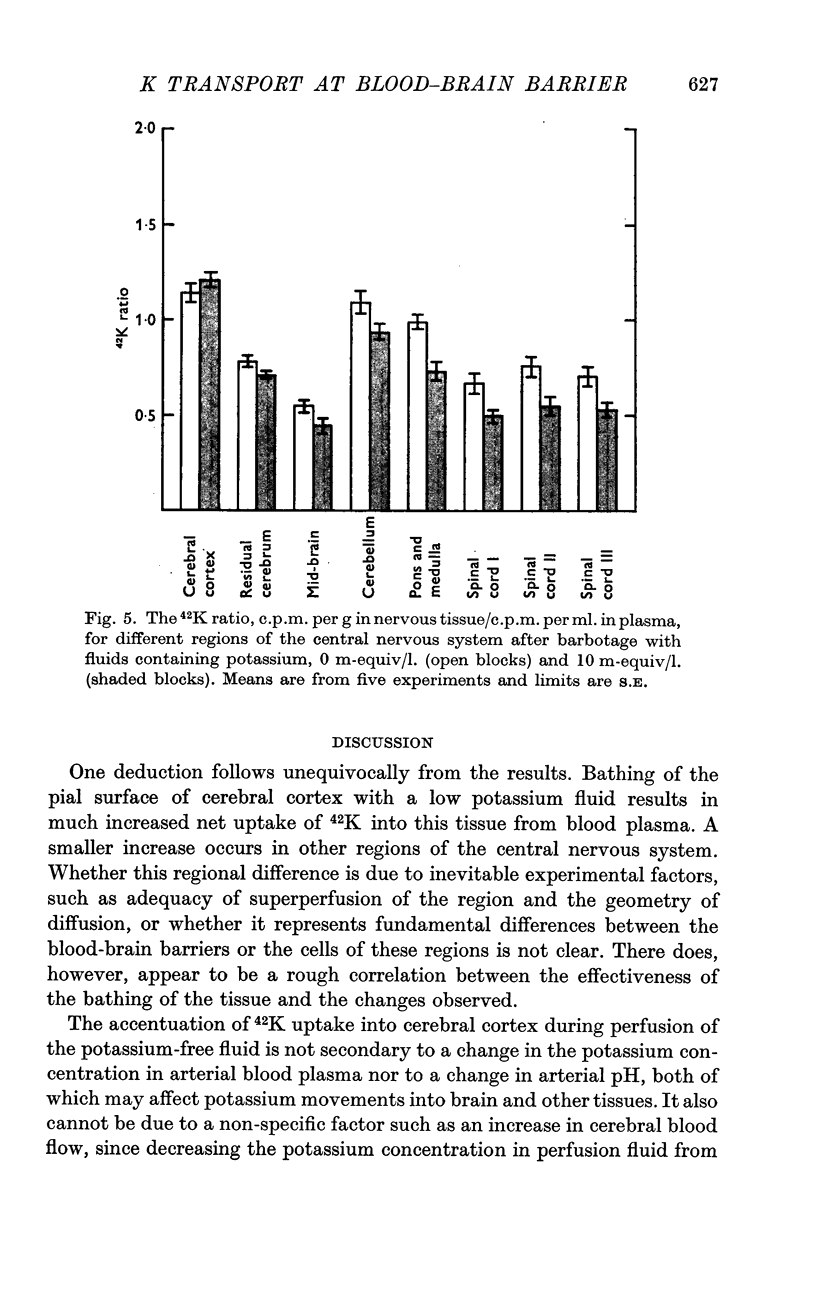
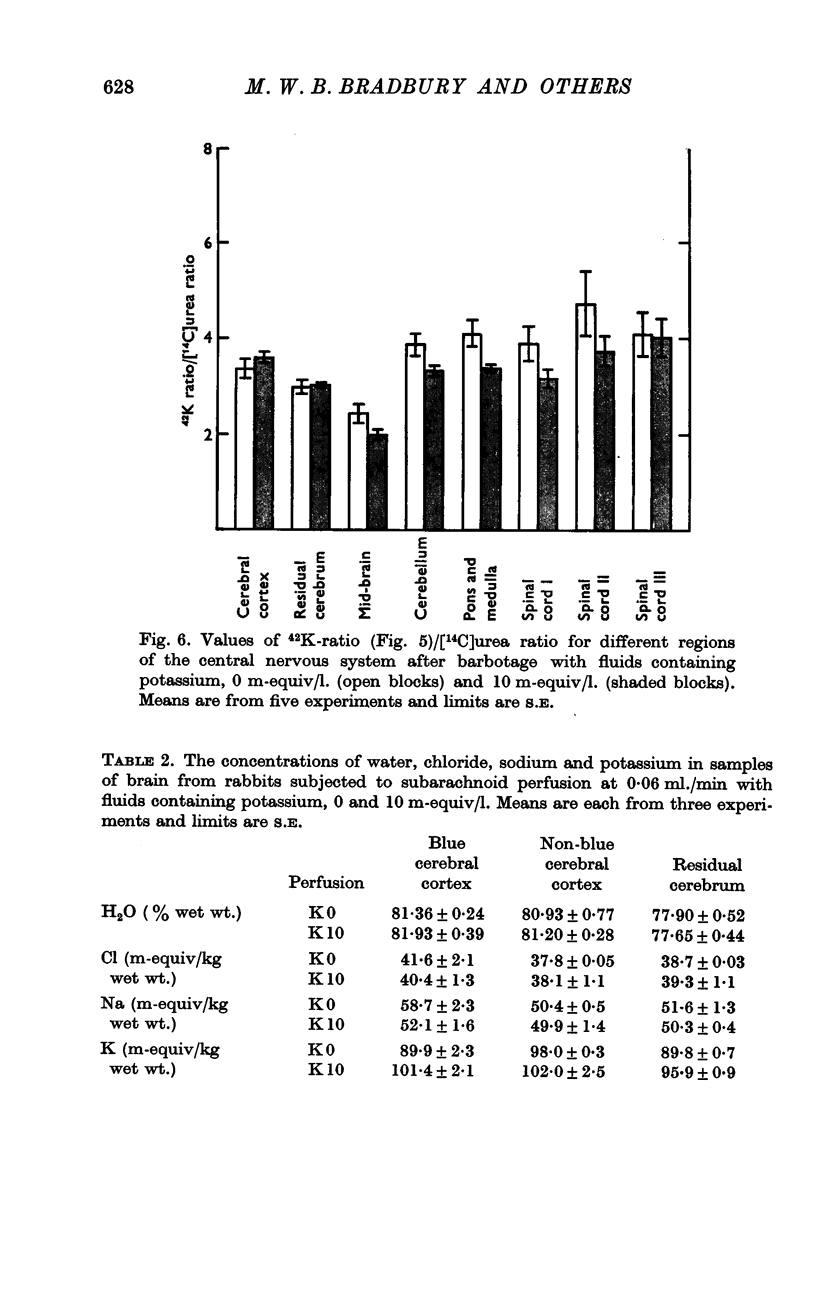
4. During barbotage, the uptake of 42K into pons-medulla and spinal cord from blood plasma, the concentration in the latter being effectively kept near constant, was, at the end of 2 hr, greater when the fluid contained potassium, 0 m=-equiv/l. rather than 10 m-equiv/l. Thus the ratio, c.p.m per g brain/c.p.m. per ml. plasma was (0·99 ± 0·04 (+36%) as against 0·73 ± 0·05 for pons-medulla where the difference was greatest.
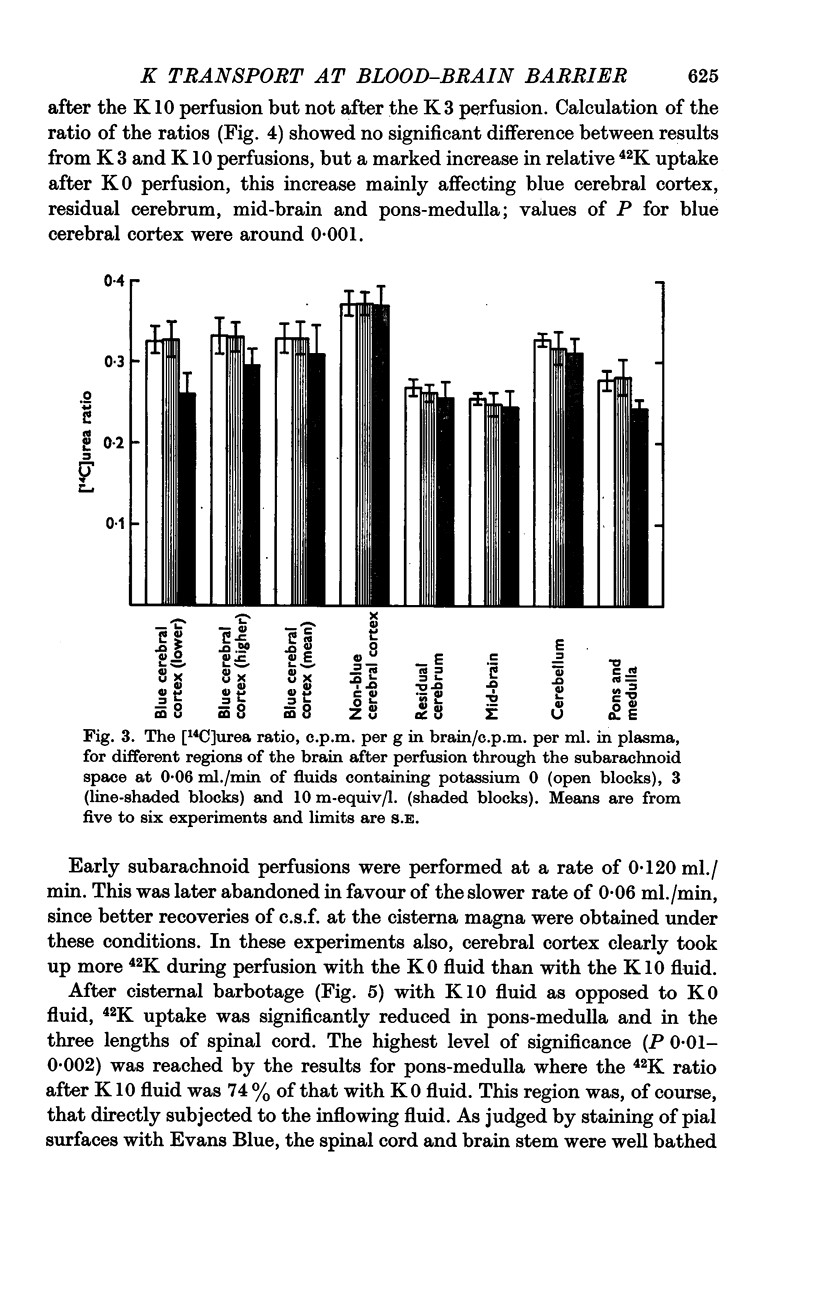
5. Simultaneous measurements of the entry of [14C]urea from blood to different regions of the central nervous system revealed no significant differences due to the differing concentrations of potassium imposed by either barbotage or subarachnoid perfusion. This appears to exclude a non-specific cause for the changes in 42K uptake, an example of which might be a changing blood flow.
6. Reasons are given for supposing that the big increase in 42K uptake due to the potassium-free fluid must be due to events occurring at the blood—brain barrier. This might be some form of interaction, possibly the single file effect, such that a low potassium concentration in the interstitial fluid potentiates 42K influx across the blood—brain barrier. Alternatively it might be due to a low potassium concentration in this fluid greatly reducing active potassium movement from interstitial fluid to blood. The former explanation would conform neatly with the present results; but the latter would additionally be compatible with other evidence concerning the homoeostasis of potassium concentration in c.s.f. and the interstitial fluid of brain.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBURY M. W., COXON R. V. The penetration of urea into the central nervous system at high blood levels. J Physiol. 1962 Oct;163:423–435. doi: 10.1113/jphysiol.1962.sp006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Davson H. The transport of potassium between blood, cerebrospinal fluid and brain. J Physiol. 1965 Nov;181(1):151–174. doi: 10.1113/jphysiol.1965.sp007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Kleeman C. R. Stability of the potassium content of cerebrospinal fluid and brain. Am J Physiol. 1967 Aug;213(2):519–528. doi: 10.1152/ajplegacy.1967.213.2.519. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Stulcová B. Efflux mechanism contributing to the stability of the potassium concentration in cerebrospinal fluid. J Physiol. 1970 Jun;208(2):415–430. doi: 10.1113/jphysiol.1970.sp009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Wilson J. Perfusion of the subarachnoid space from above the corpus callosum to the cisterna magna--transport of potassium at the blood-brain barrier. J Physiol. 1971 Oct;218 (Suppl):8P–9P. [PubMed] [Google Scholar]
- Cohen M. W., Gerschenfeld H. M., Kuffler S. W. Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol. 1968 Jul;197(2):363–380. doi: 10.1113/jphysiol.1968.sp008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H., Rall D. P. Regulation of cerebrospinal fluid [K+] in the spiny dogfish, Squalus acanthias. Comp Biochem Physiol. 1967 May;21(2):431–434. doi: 10.1016/0010-406x(67)90805-5. [DOI] [PubMed] [Google Scholar]
- Fenstermacher J. D., Li C. L., Levin V. A. Extracellular space of the cerebral cortex of normothermic and hypothermic cats. Exp Neurol. 1970 Apr;27(1):101–114. doi: 10.1016/0014-4886(70)90205-0. [DOI] [PubMed] [Google Scholar]
- HARRIS E. J. Permeation and diffusion of K ions in frog muscle. J Gen Physiol. 1957 Sep 20;41(1):169–195. doi: 10.1085/jgp.41.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELD D., FENCL V., PAPPENHEIMER J. R. ELECTRICAL POTENTIAL OF CEREBROSPINAL FLUID. J Neurophysiol. 1964 Sep;27:942–959. doi: 10.1152/jn.1964.27.5.942. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M. Ion transport across the frog posterior choroid plexus. Brain Res. 1970 Oct 13;23(2):302–304. doi: 10.1016/0006-8993(70)90057-0. [DOI] [PubMed] [Google Scholar]