Abstract
1. The uptake, esterification and transport of [14C]oleic acid were studied using sacs of rat everted small intestine incubated in 25 ml. of a buffered mixture of sodium taurocholate, glyceryl mono-oleate and 14C-labelled oleic acid in micellar form.
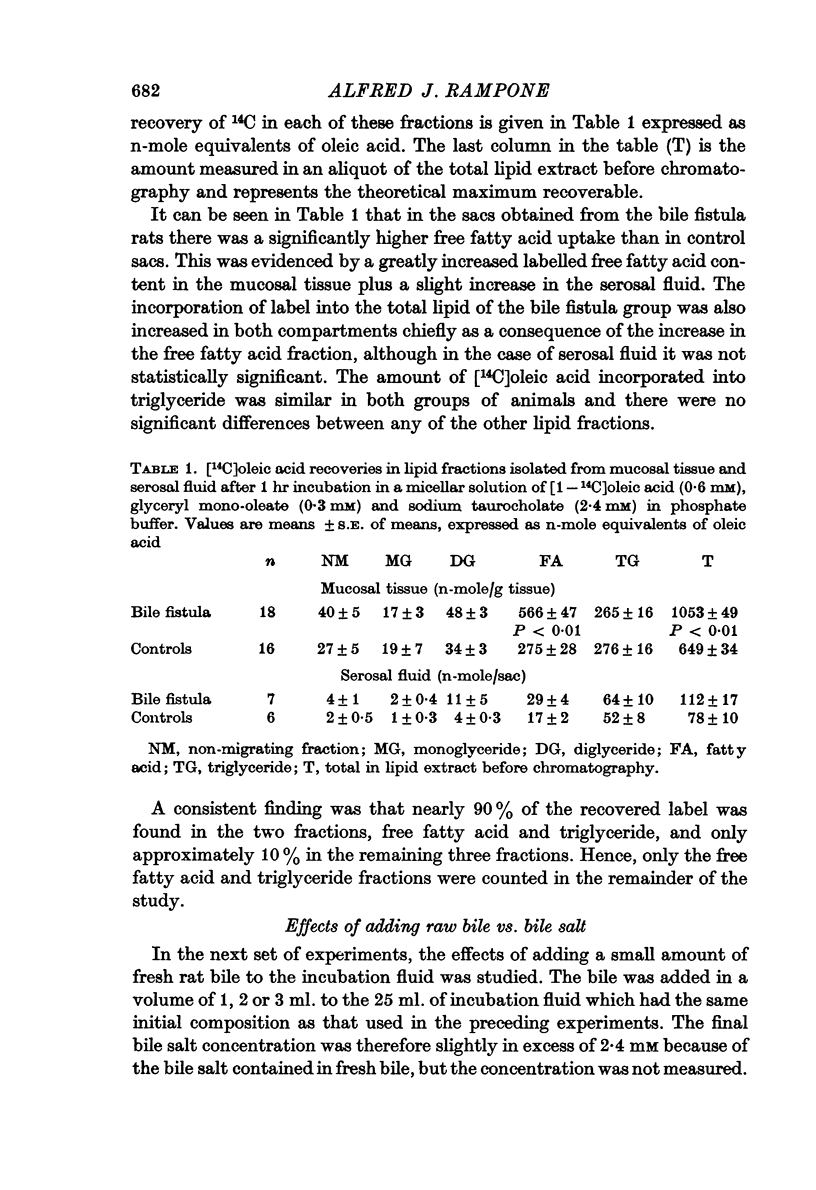
2. Intestine obtained from bile fistula rats (bile duct cannulated 48 hr previously) showed elevated rates of 14C uptake into the tissue total lipid compared with sham-operated controls.
3. Nearly all of the excess 14C uptake in the bile fistula group was in the form of free fatty acid. Both groups showed similar rates of [14C]oleic acid incorporation into tissue triglyceride and also similar, though small, amounts transported into the serosal fluid.
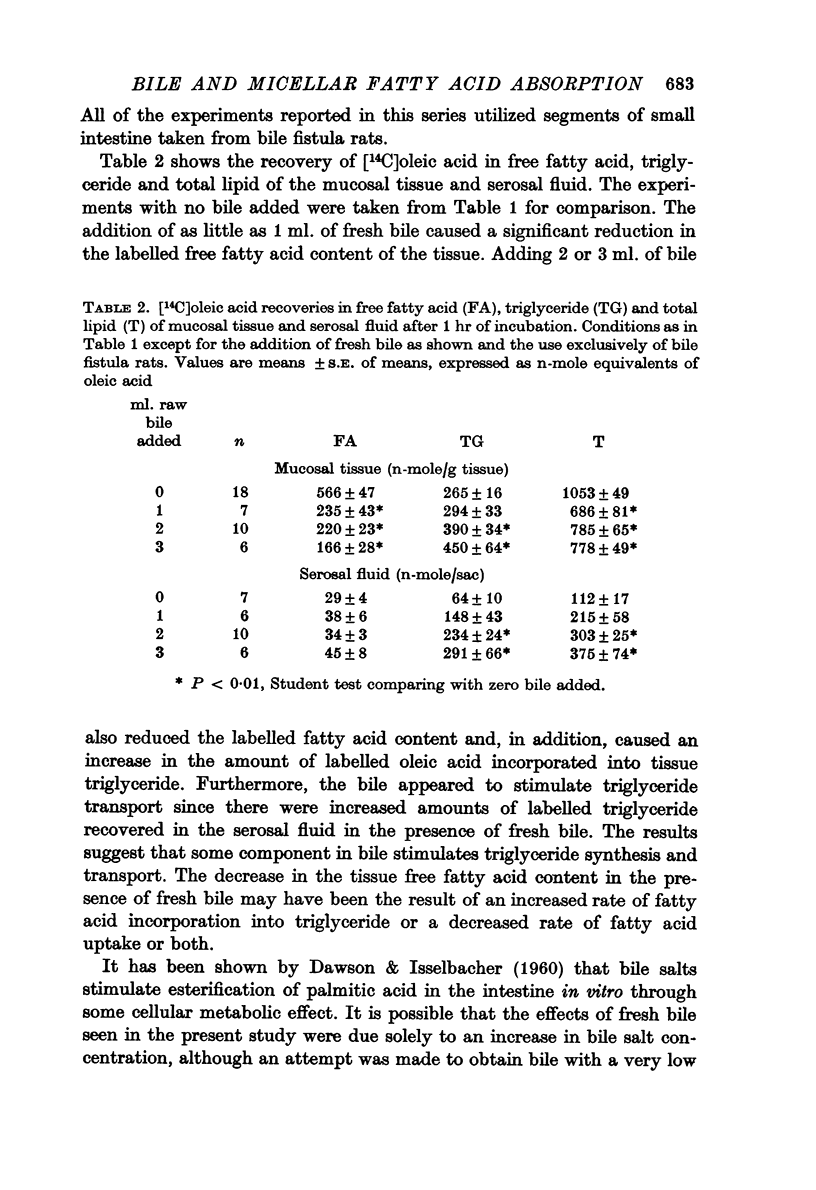
4. In further experiments using intestine from bile fistula rats the addition of 1 ml. of fresh rat bile to the incubation mixture reduced the 14C uptake to approximately control levels. The addition of 2-3 ml. of fresh bile similarly reduced the uptake and increased 14C incorporation into the triglycerides of mucosal tissue and serosal fluid.
5. These responses were not entirely the result of the bile salts contained in fresh bile since increasing the taurocholate concentration per se caused uptake, esterification and transport all to increase. In the presence of the higher taurocholate concentration the addition of fresh bile still caused a decrease in 14C uptake.
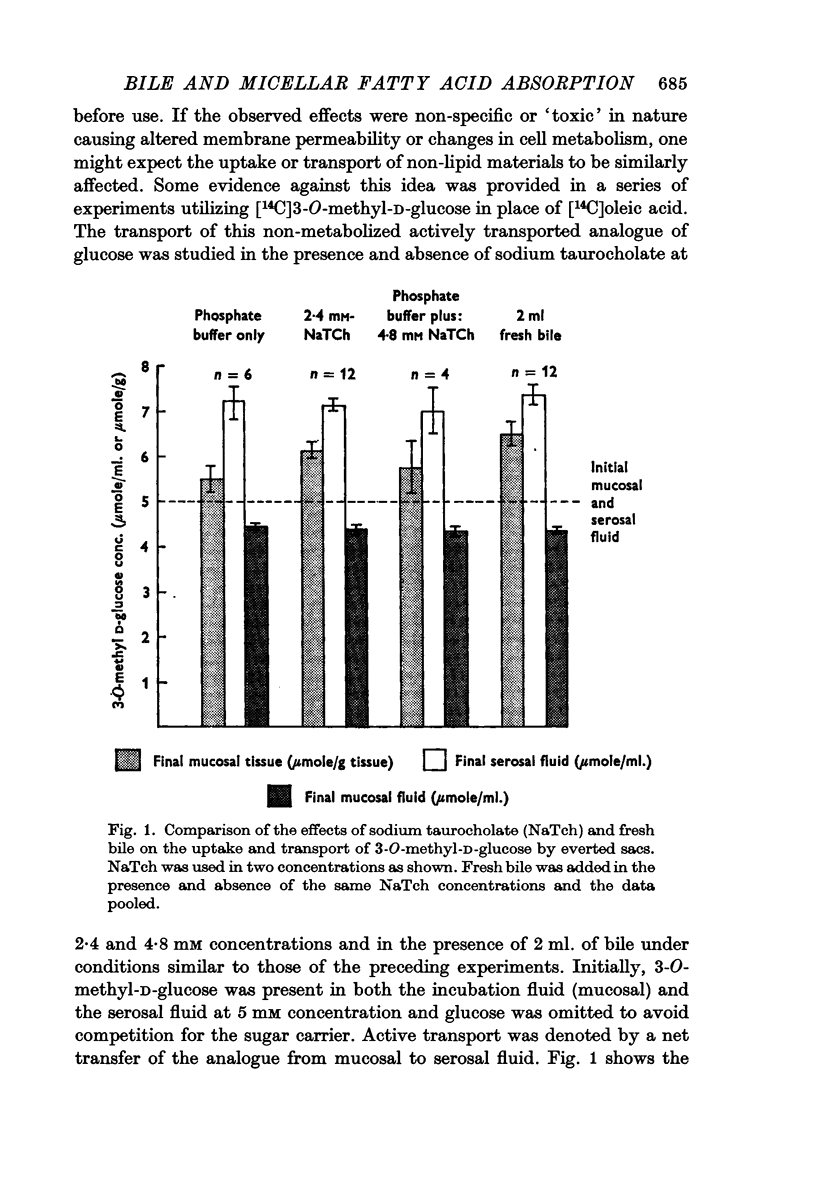
6. There was no significant effect of either fresh bile or taurocholate on the transport of the 3-O-methyl analogue of D-glucose under comparable conditions.
7. It is concluded that raw bile contains one or more components other than bile salts which may be important in determining fatty acid absorption.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgström B. The dimensions of the bile salt micelle. Measurements by gel filtration. Biochim Biophys Acta. 1965 Jul 7;106(1):171–183. doi: 10.1016/0005-2760(65)90105-0. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., GLENN J. E. Urinary recovery of 3-methylglucose administered to rats. Am J Physiol. 1957 Jan;188(1):159–162. doi: 10.1152/ajplegacy.1956.188.1.159. [DOI] [PubMed] [Google Scholar]
- DAWSON A. M., ISSELBACHER K. J. Studies on lipid metabolism in the small intestine with observations on the role of bile salts. J Clin Invest. 1960 May;39:730–740. doi: 10.1172/JCI104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Faust R. G., Wu S. M. The action of bile salts on fluid and glucose movement by rat and hamster jejunum, in vitro. J Cell Physiol. 1965 Jun;65(3):435–448. doi: 10.1002/jcp.1030650318. [DOI] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROEM B. THE INTRALUMINAL PHASE OF FAT DIGESTION IN MAN: THE LIPID CONTENT OF THE MICELLAR AND OIL PHASES OF INTESTINAL CONTENT OBTAINED DURING FAT DIGESTION AND ABSORPTION. J Clin Invest. 1964 Feb;43:247–257. doi: 10.1172/JCI104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROM B. Physico-chemical state of lipids in intestinal content during their digestion and absorption. Fed Proc. 1962 Jan-Feb;21:43–50. [PubMed] [Google Scholar]
- Hofmann A. F. A physicochemical approach to the intraluminal phase of fat absorption. Gastroenterology. 1966 Jan;50(1):56–64. [PubMed] [Google Scholar]
- JOHNSTON J. M., BORGSTROEM B. THE INTESTINAL ABSORPTION AND METABOLISM OF MICELLAR SOLUTIONS OF LIPIDS. Biochim Biophys Acta. 1964 Aug 5;84:412–423. doi: 10.1016/0926-6542(64)90005-8. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D. Does bile acid secretion determine canalicular bile production in rats? Am J Physiol. 1971 Mar;220(3):667–673. doi: 10.1152/ajplegacy.1971.220.3.667. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Yalovsky M., Kessler J. I. The in vitro uptake and kinetics of release of palmitic acid and taurodeoxycholate from hamster small intestinal segments. Biochim Biophys Acta. 1971 Feb 2;225(2):335–346. doi: 10.1016/0005-2736(71)90227-6. [DOI] [PubMed] [Google Scholar]
- Rampone A. J. Intestinal absorption of micellar lipid in normal and bile deficient rats. Proc Soc Exp Biol Med. 1970 Dec;135(3):666–670. doi: 10.3181/00379727-135-35117. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. The absorption of micellar lipid into the lymph of unanaesthetised rats. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):130–138. doi: 10.1113/expphysiol.1967.sp001894. [DOI] [PubMed] [Google Scholar]
- Roy C. C., Dubois R. S., Philippon F. Inhibition by bile salts of the jejunal transport of 3-O-methyl glucose. Nature. 1970 Mar 14;225(5237):1055–1056. doi: 10.1038/2251055a0. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., LANDAU B. R. Specificity of sugar transport by the intestine of the hamster. Am J Physiol. 1960 Jan;198:99–102. doi: 10.1152/ajplegacy.1960.198.1.99. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J. P., Hamilton J. D., Dawson A. M. A physicochemical study of fat absorption in rats. Limitation of methods in vitro. Biochim Biophys Acta. 1969 Jul 29;187(1):42–52. doi: 10.1016/0005-2760(69)90131-3. [DOI] [PubMed] [Google Scholar]


