Abstract
1. The spectral sensitivity of red-green dichromats was determined using heterochromatic flicker photometric matches (25-30 c/s) on the fovea. These matches are upset after a bright bleach and consequently the spectral sensitivity is altered.
2. Preliminary experiments indicate that under the conditions in which these experiments were performed, the blue cone mechanism of deuteranopes and protanopes cannot follow 20 c/s flicker. If dichromats lack one of the normal pigments then the upset of these matches monitors the change in spectral sensitivity of a single mechanism.
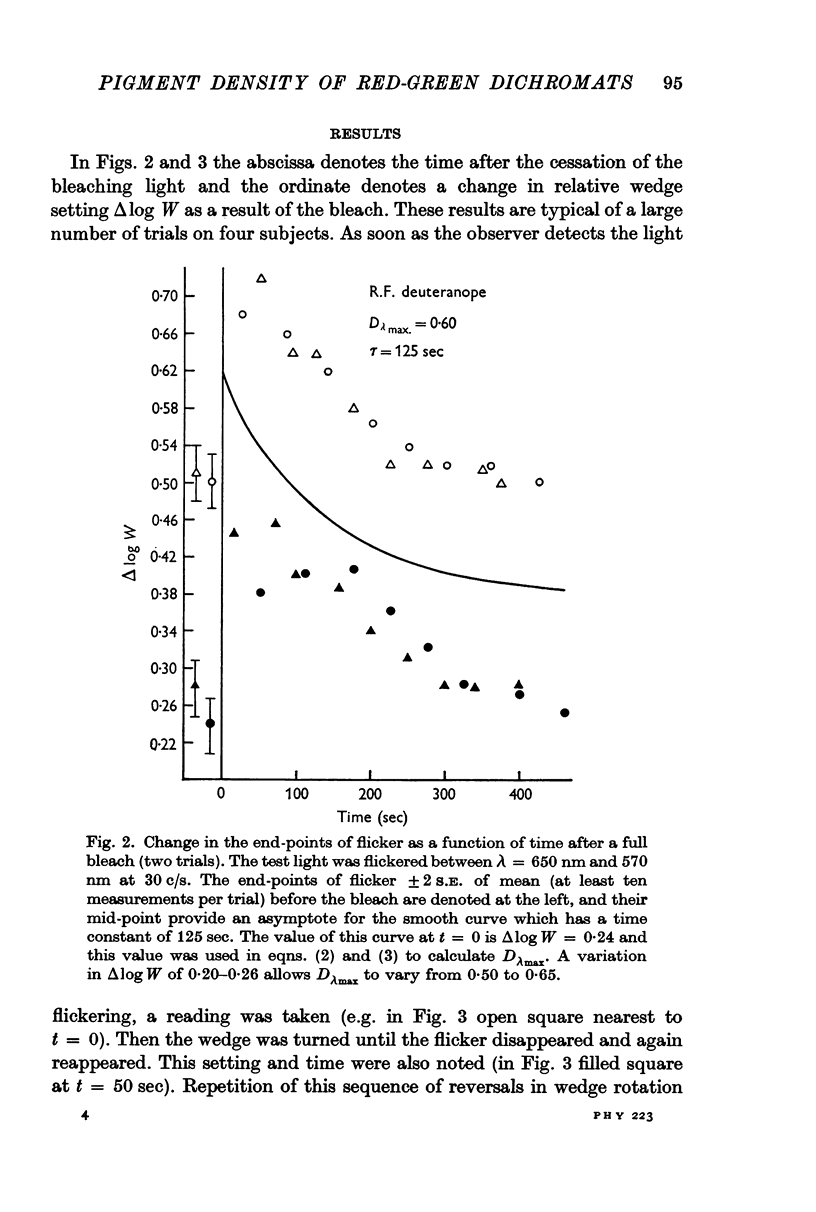
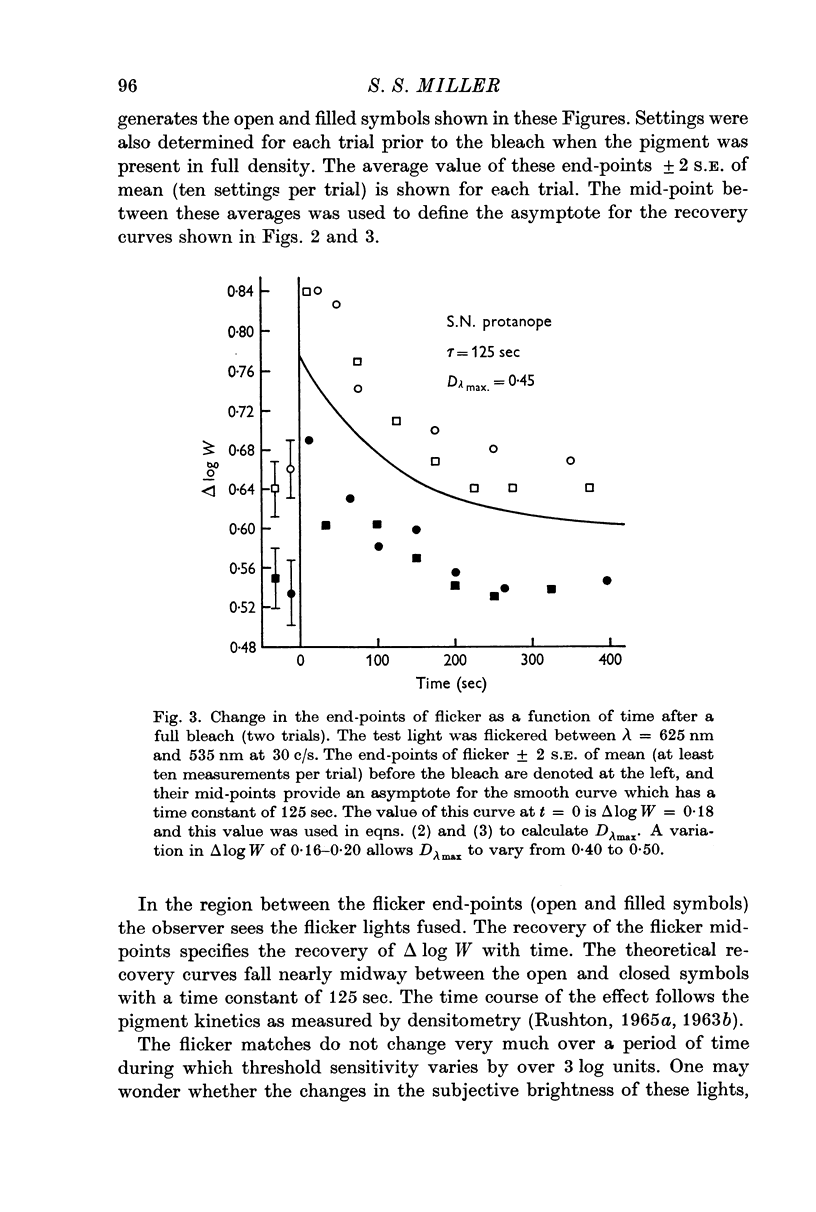
3. After a bleach which removes all the cone pigments, the spectral sensitivity recovers with the time course of pigment kinetics as measured by densitometry.
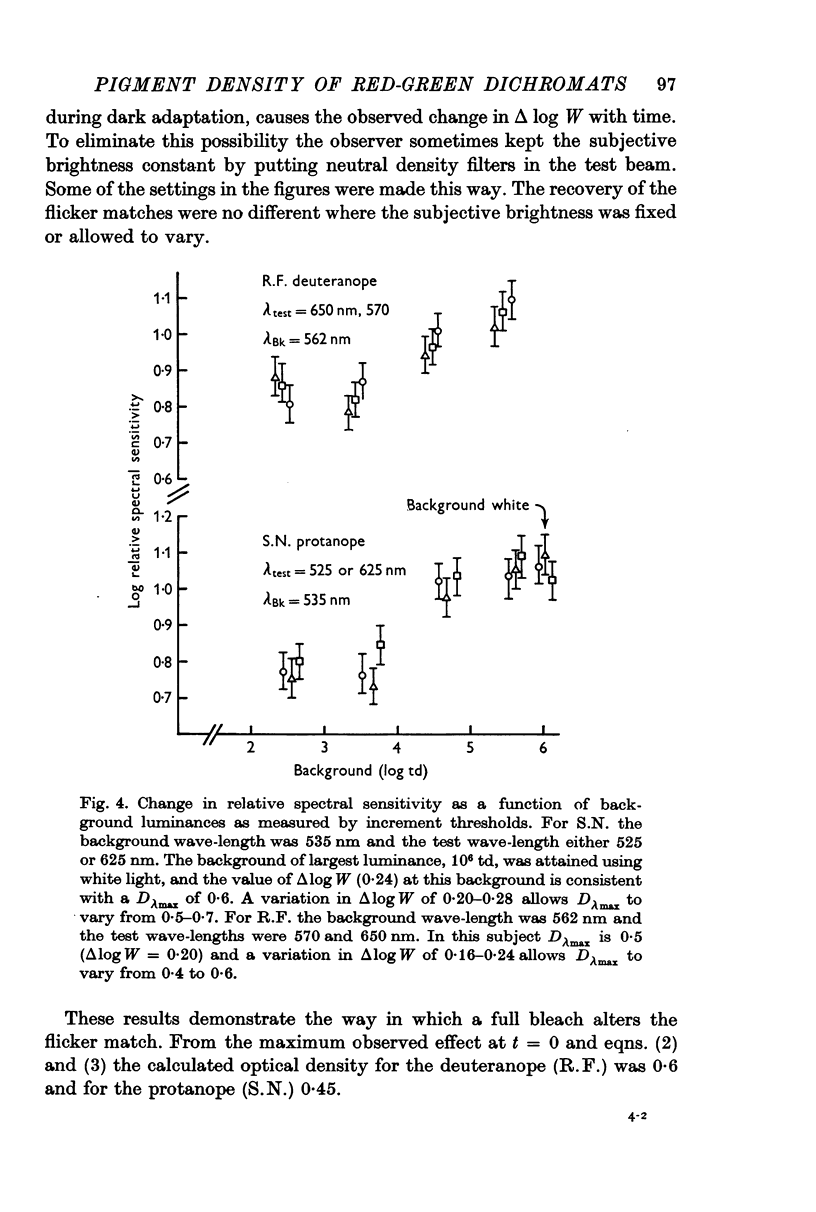
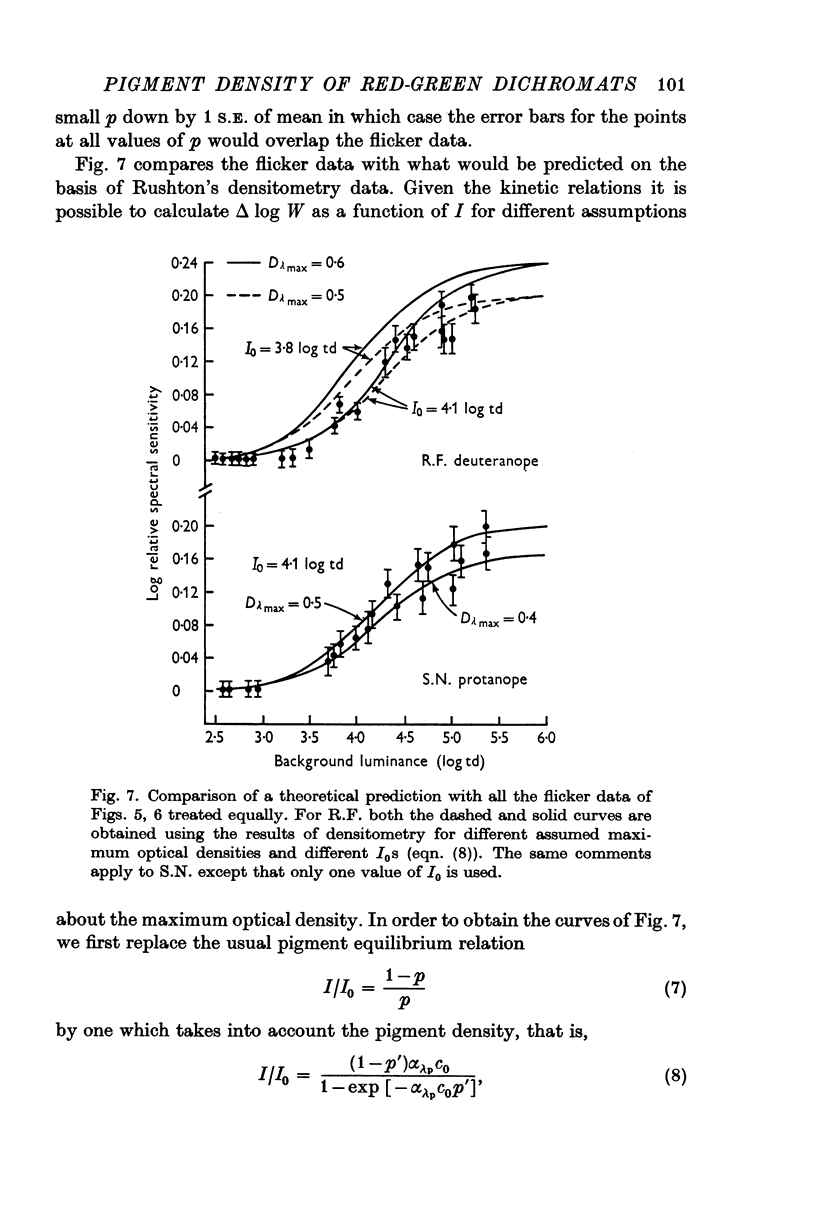
4. An intense background also changes the relative spectral sensitivity of the dichromats. On real equilibrium backgrounds, the changes in spectral sensitivity follow those predicted by the pigment changes measured by densitometry. The predicted changes are obtained by modifying the Rushton equilibrium equation to take into account the density of pigment.
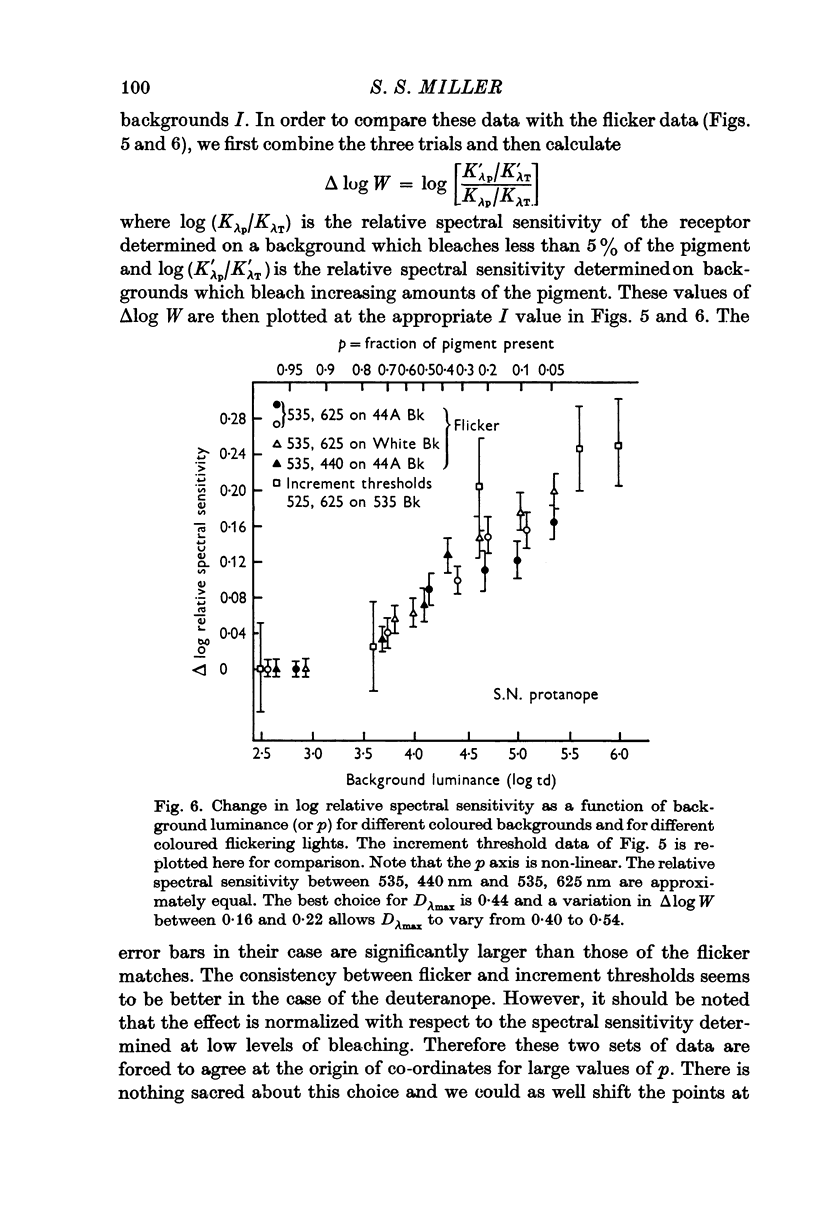
5. The relationship of these changes to the luminance of the background is independent of the colour of the background light.
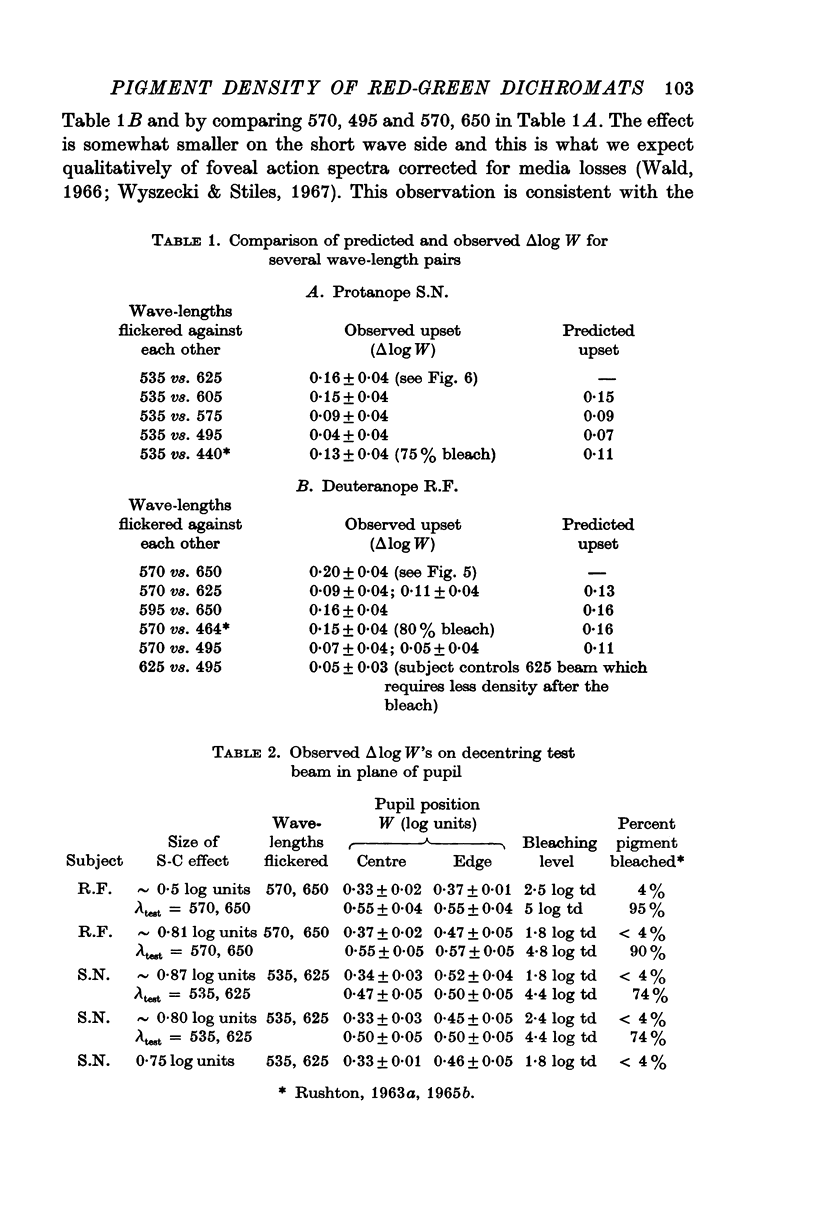
6. In contradistinction the effect is dependent on the colour of the lights which were flickered. These experiments indicate that a narrowing of the spectral sensitivity curves takes place on both sides of the dichromats' λmax.
7. The change in relative spectral sensitivity as a function of background intensity was also determined by increment threshold measurements. These changes can be expressed in terms of deviations from Weber's law (ΔI/I = const.) if ΔI and I represent the number of chromophores destroyed by the test and background.
8. The relative spectral sensitivity of the dichromat was changed by decentering the point of pupil entry. This upset was abolished by bleaching. The size of the upset was correlated with the magnitude of the S—C I effect.
9. Given the hypothesis of pigment density (self-screening), the results of expts. (3)-(8) are consistent and allow the calculation of a maximum optical density for those pigments which underlie the dichromats' long-wave mechanism. For the deuteranope a Dλmax of 0·5-0·6 is calculated and for the protanope a Dλmax of 0·4-0·5 is obtained.
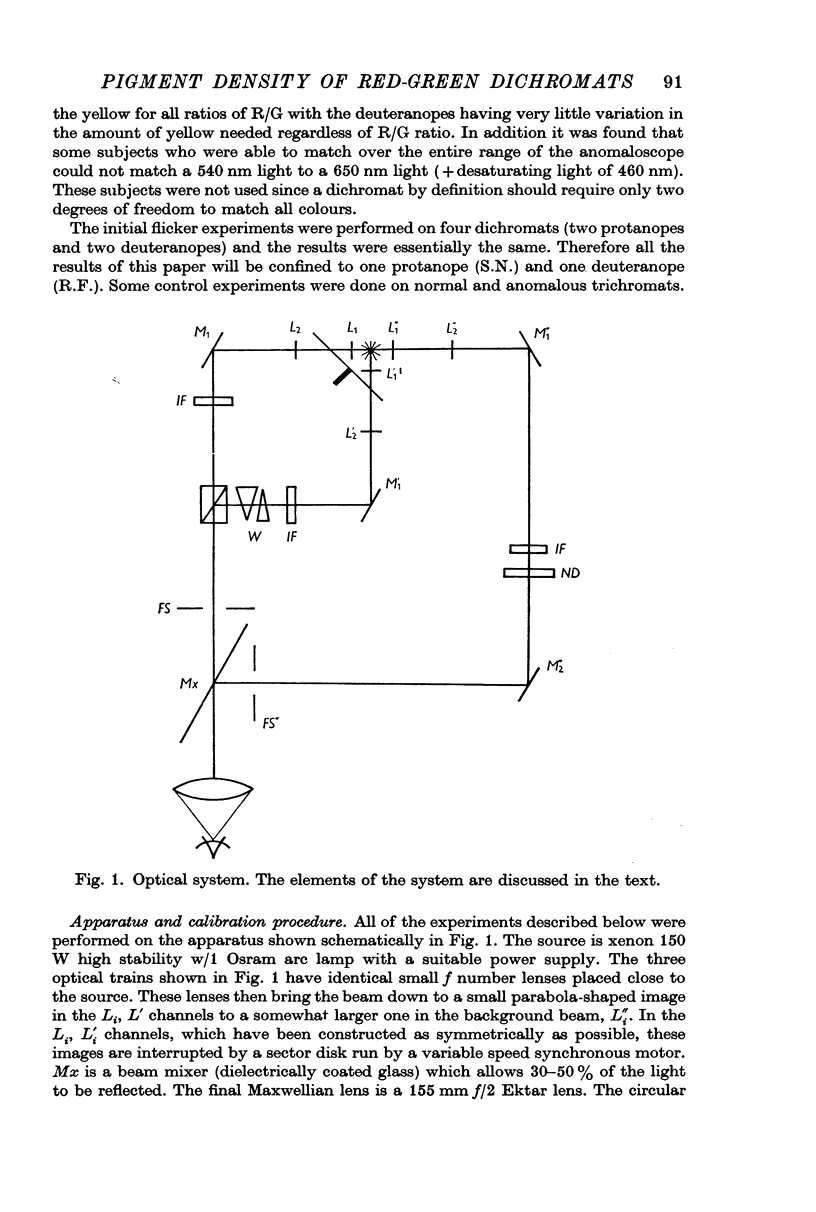
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINDLEY G. S. The effects on colour vision of adaptation to very bright lights. J Physiol. 1953 Nov 28;122(2):332–350. doi: 10.1113/jphysiol.1953.sp005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley G. S., Du Croz J. J., Rushton W. A. The flicker fusion frequency of the blue-sensitive mechanism of colour vision. J Physiol. 1966 Mar;183(2):497–500. doi: 10.1113/jphysiol.1966.sp007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobelle W. H., Marks W. B., MacNichol E. F., Jr Visual pigment density in single primate foveal cones. Science. 1969 Dec 19;166(3912):1508–1510. doi: 10.1126/science.166.3912.1508. [DOI] [PubMed] [Google Scholar]
- ENOCH J. M., STILES W. S. The colour change of monochromatic light with retinal angle of incidence. Optom Wkly. 1961 Oct;8(52):329–358. doi: 10.1080/713826396. [DOI] [PubMed] [Google Scholar]
- Feltz A., Mallart A. Ionic permeability changes induced by some cholinergic agonists on normal and denervated frog muscles. J Physiol. 1971 Oct;218(1):101–116. doi: 10.1113/jphysiol.1971.sp009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G. Sinusoidal flicker characteristics of the color-sensitive mechanisms of the eye. Vision Res. 1969 May;9(5):591–601. doi: 10.1016/0042-6989(69)90021-2. [DOI] [PubMed] [Google Scholar]
- Hood C., Rushton W. A. The Florida retinal densitometer. J Physiol. 1971 Aug;217(1):213–229. doi: 10.1113/jphysiol.1971.sp009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Rushton W. A. The red-green pigments of normal vision. Vision Res. 1971 Oct;11(10):1045–1056. doi: 10.1016/0042-6989(71)90111-8. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Rushton W. A. Visual pigments in dichromats. Vision Res. 1971 Oct;11(10):1033–1043. doi: 10.1016/0042-6989(71)90110-6. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. A CONE PIGMENT IN THE PROTANOPE. J Physiol. 1963 Sep;168:345–359. doi: 10.1113/jphysiol.1963.sp007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. A FOVEAL PIGMENT IN THE DEUTERANOPE. J Physiol. 1965 Jan;176:24–37. doi: 10.1113/jphysiol.1965.sp007532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. CONE PIGMENT KINETICS IN THE DEUTERANOPE. J Physiol. 1965 Jan;176:38–45. doi: 10.1113/jphysiol.1965.sp007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. CONE PIGMENT KINETICS IN THE PROTANOPE. J Physiol. 1963 Sep;168:374–388. doi: 10.1113/jphysiol.1963.sp007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALRAVEN P. L., BOUMAN M. A. Relation between directional sensitivity and spectral response curves in human cone vision. J Opt Soc Am. 1960 Aug;50:780–784. doi: 10.1364/josa.50.000780. [DOI] [PubMed] [Google Scholar]
- Wald G. Defective color vision and its inheritance. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1347–1363. doi: 10.1073/pnas.55.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. D. The breakdown of a colour match with high intensities of adaptation. J Physiol. 1936 Jun 10;87(1):23–33. doi: 10.1113/jphysiol.1936.sp003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T., Wald G. Photochemistry of lodopsin. Nature. 1967 May 6;214(5088):566–571. doi: 10.1038/214566a0. [DOI] [PubMed] [Google Scholar]


