Abstract
1. End-plate currents have been studied in glycerol-treated frog sartorius nerve—muscle preparations with the voltage clamp technique.
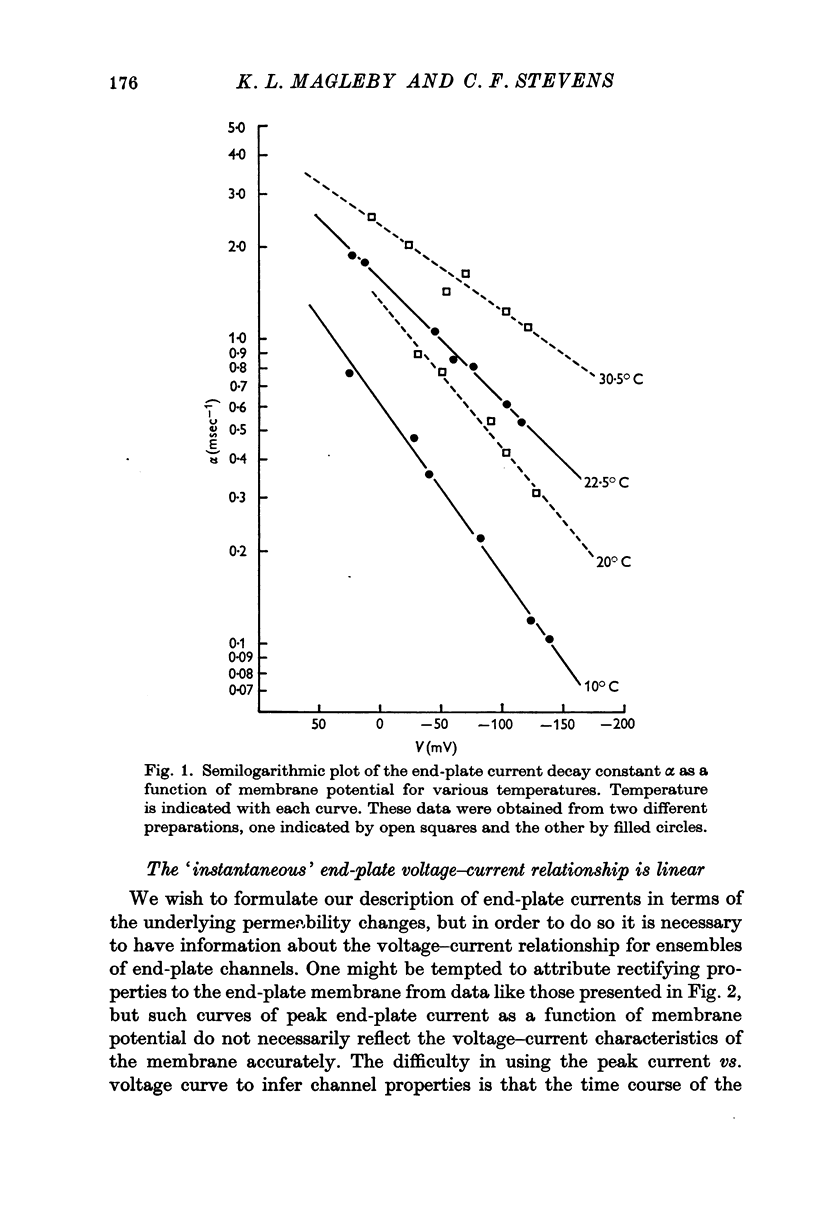
2. The effects of temperature on the decay rate of end-plate currents were investigated over a temperature range from 10 to 30·5° C. The Q10 for the decay constant of end-plate currents depends somewhat on membrane potential; at — 100 mV the decay constant has a Q10 of 2·7.
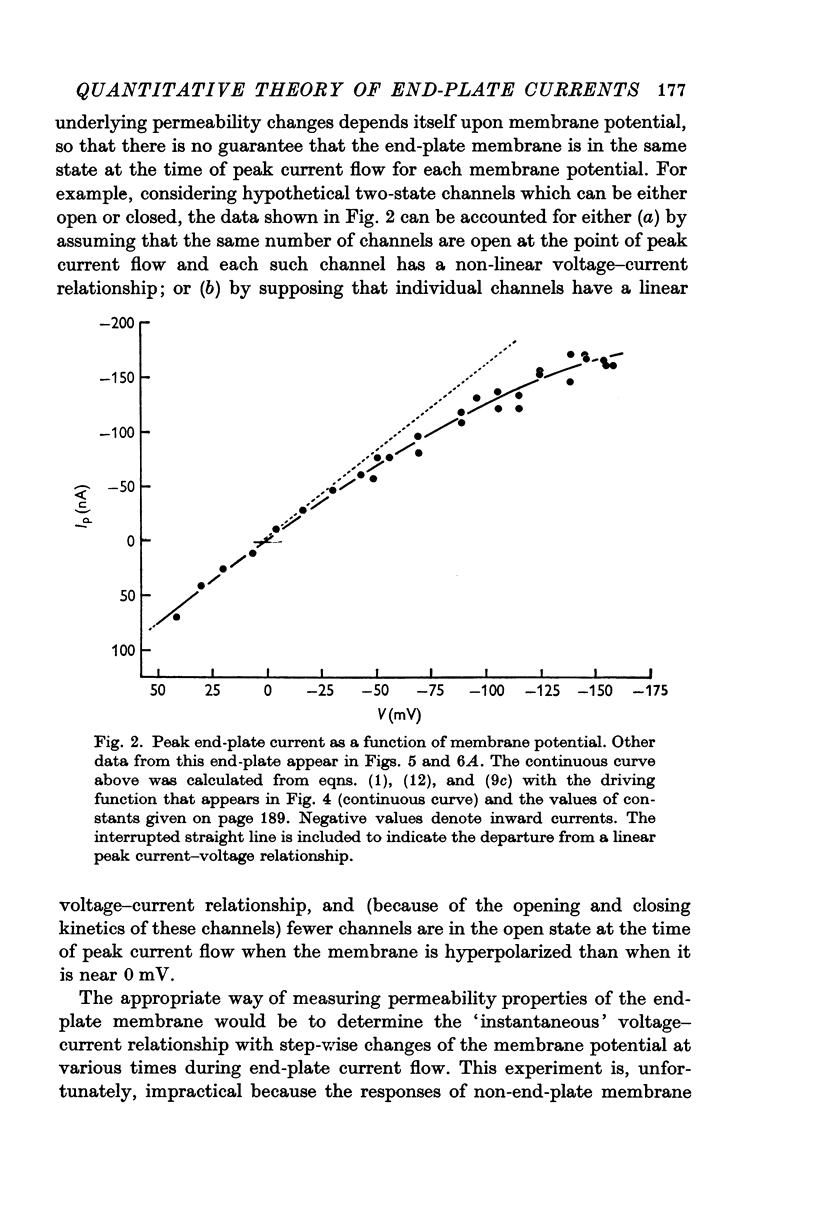
3. Peak end-plate current depends non-linearly on membrane potential with a decreasing slope conductance associated with hyperpolarization.
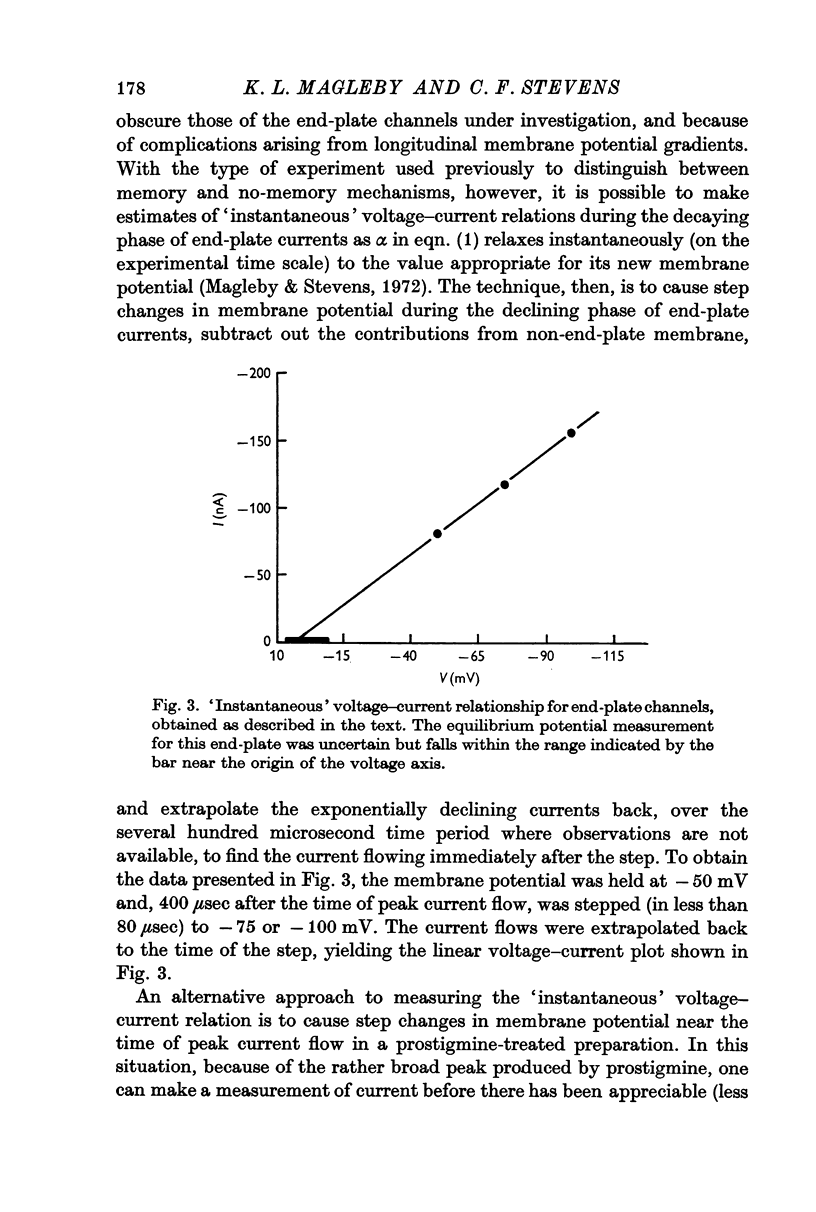
4. The `instantaneous' voltage—current relationship for end-plate channels was determined by causing step changes in membrane potential during end-plate current flow. This relationship appears to be linear.
5. The interaction of acetylcholine with its receptor is viewed as being analogous to the first step in enzymic catalysis. On this view, acetylcholine binds to its receptor and induces a conformational change which is responsible for opening end-plate channels. By analogy to the first steps in the catalytic sequence of enzymes, the binding step is very rapid, almost diffusion-limited, and the conformational change is rate-limiting.
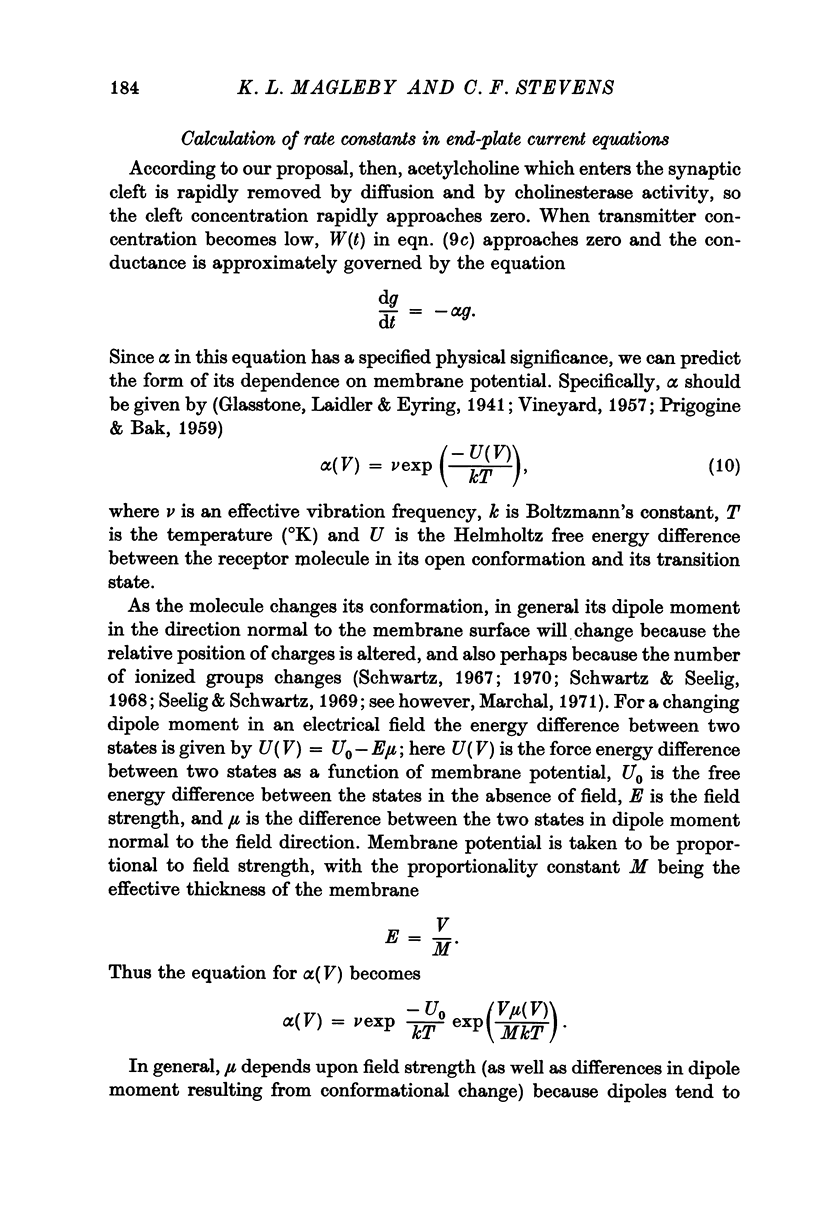
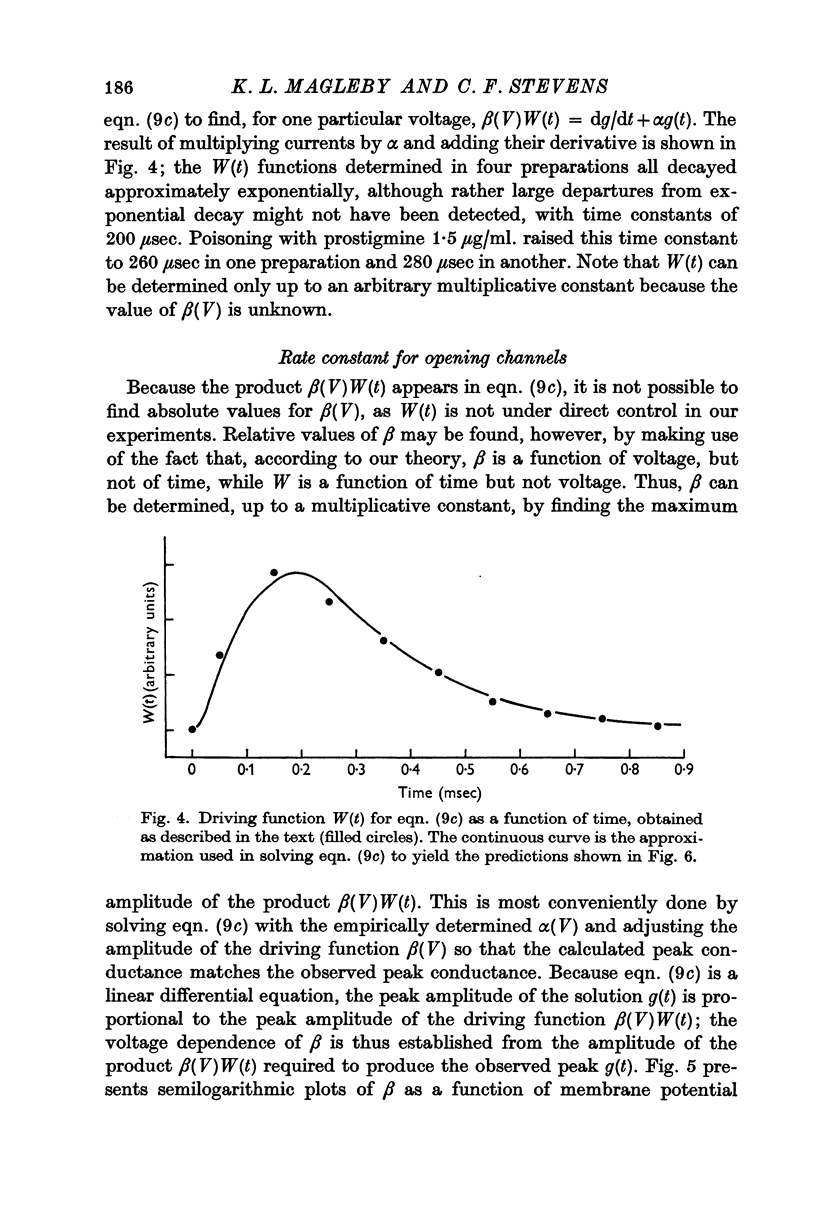
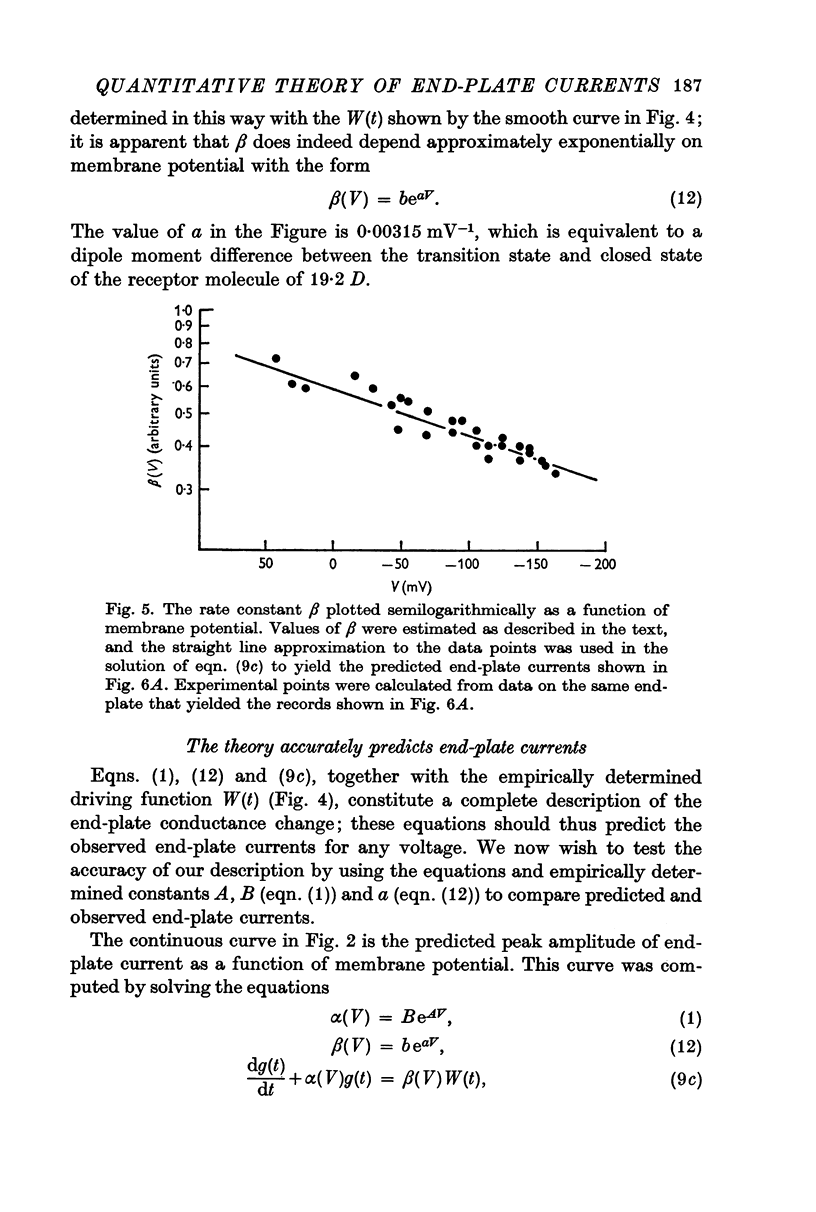
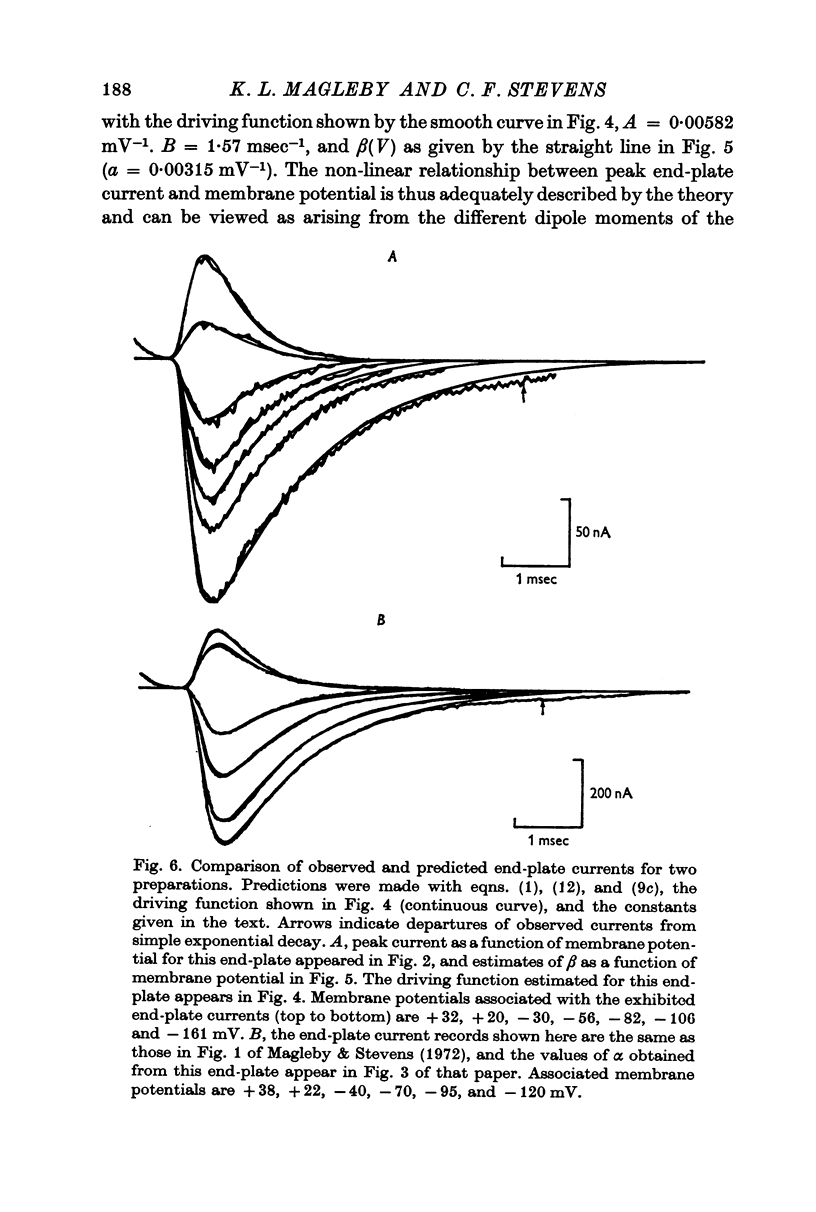
6. Equations describing this process have been derived. Expressions for the rate constants have also been derived by considering changing dipole moments of the transmitter-receptor complex associated with the conformational change. As the transmitter-receptor complex is in the membrane field, different conformational states have different energies, and the rate of conformational change thus depends on membrane potential. The equations thus derived are shown to account adequately for the time course of end-plate conductance change.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Meunier J. C., Huchet M. Studies on the cholinergic receptor protein of Electrophorus electricus. I. An assay in vitro for the cholinergic receptor site and solubilization of the receptor protein from electric tissue. Mol Pharmacol. 1971 Sep;7(5):538–553. [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock P. B. Relaxation methods and enzymology. Biochimie. 1971;53(2):161–172. doi: 10.1016/s0300-9084(71)80048-2. [DOI] [PubMed] [Google Scholar]
- Del Rosario E. J., Hammes G. G. Relaxation spectra of ribonuclease. VII. The interaction of ribonuclease with uridine 2',3'-cyclic phosphate. J Am Chem Soc. 1970 Mar 25;92(6):1750–1753. doi: 10.1021/ja00709a056. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Erman J. E., Hammes G. G. Relaxation spectra of ribonuclease. IV. The interaction of ribonuclease with cytidine 2':3'-cyclic phosphate. J Am Chem Soc. 1966 Dec 5;88(23):5607–5614. doi: 10.1021/ja00975a046. [DOI] [PubMed] [Google Scholar]
- FURUKAWA T. Properties of the procaine end-plate potential. Jpn J Physiol. 1957 Sep 30;7(3):199–212. doi: 10.2170/jjphysiol.7.199. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G. Relaxation spectrometry of biological systems. Adv Protein Chem. 1968;23:1–57. doi: 10.1016/s0065-3233(08)60399-x. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Simplicio J. Relaxation spectra of pyruvate kinase. Biochim Biophys Acta. 1970 Sep 16;212(3):428–433. doi: 10.1016/0005-2744(70)90248-2. [DOI] [PubMed] [Google Scholar]
- Holler E., Rupley J. A., Hess G. P. Kinetics of lysozyme-substrate interactions. Biochem Biophys Res Commun. 1969 Oct 22;37(3):423–429. doi: 10.1016/0006-291x(69)90932-2. [DOI] [PubMed] [Google Scholar]
- Janin J., Iwatsubo M. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. Relaxations of the allosteric equilibrium. Eur J Biochem. 1969 Dec;11(3):530–540. doi: 10.1111/j.1432-1033.1969.tb00805.x. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further observations on acetylcholine noise. Nat New Biol. 1971 Jul 28;232(30):124–126. doi: 10.1038/newbio232124b0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Eigen M., Bittman R., Voigt B. The binding of nicotinamide-adenine dinucleotide to yeast d-glyceraldehyde-3-phosphate dehydrogenase: temperature-jump relaxation studies on the mechanism of an allosteric enzyme. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1661–1667. doi: 10.1073/pnas.56.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Drug receptors and their function. Nature. 1971 May 14;231(5298):91–96. doi: 10.1038/231091a0. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Seelig J. Kinetic properties and the electric field effect of the helix-coil transition of poly(gamma-benzyl L-glutamate) determined from dielectric relaxation measurements. Biopolymers. 1968;6(9):1263–1277. doi: 10.1002/bip.1968.360060904. [DOI] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]


