Abstract
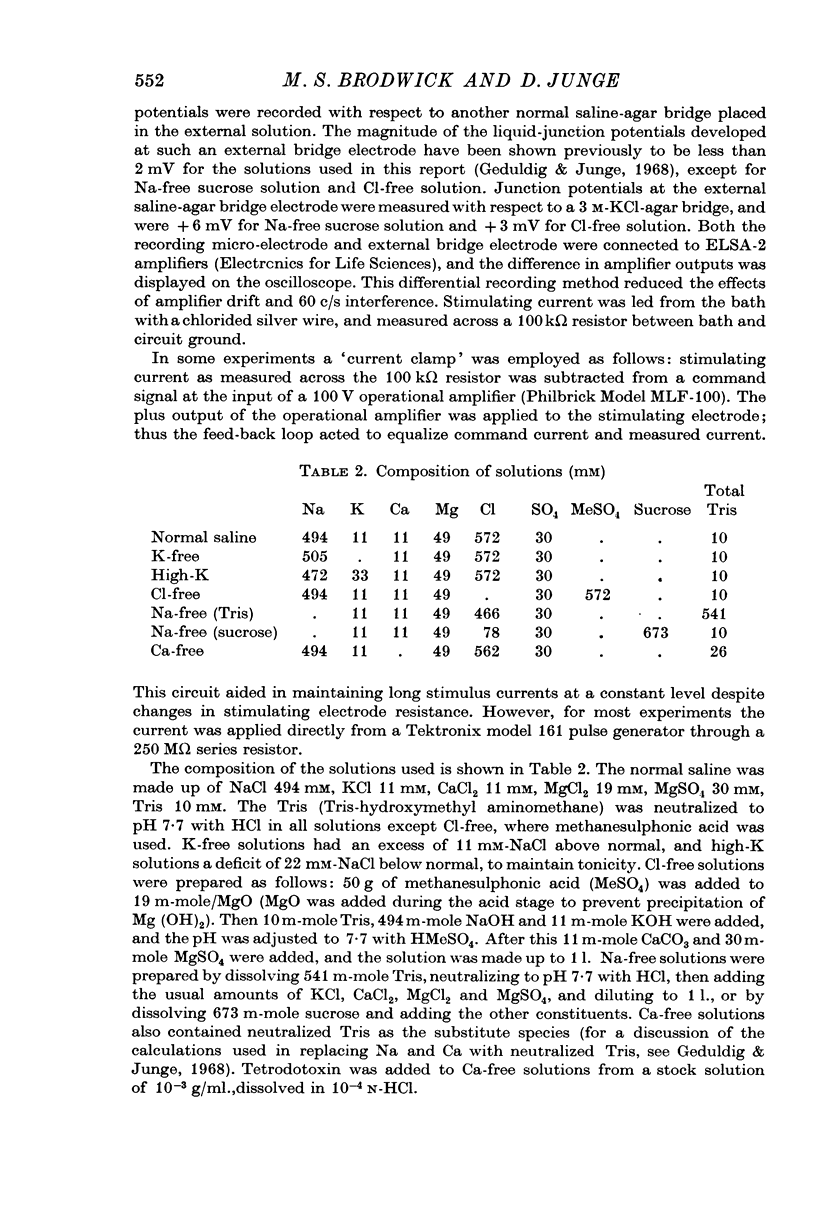
1. Intracellular records from Aplysia giant (R2) cell somata showed long lasting 4-10 mV hyperpolarizations after passage of outward current through a second intracellular electrode.
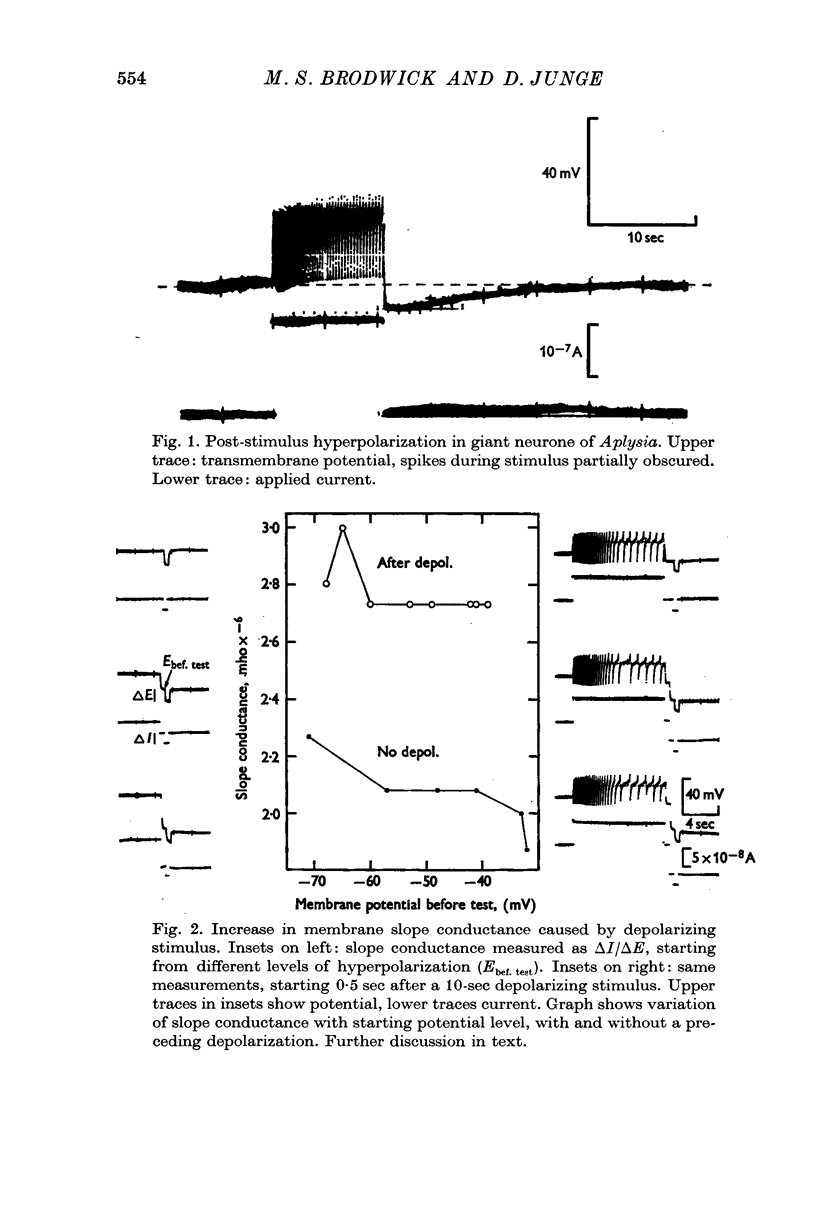
2. An increase in membrane slope conductance occurred simultaneously with the post-stimulus hyperpolarization (PSH).
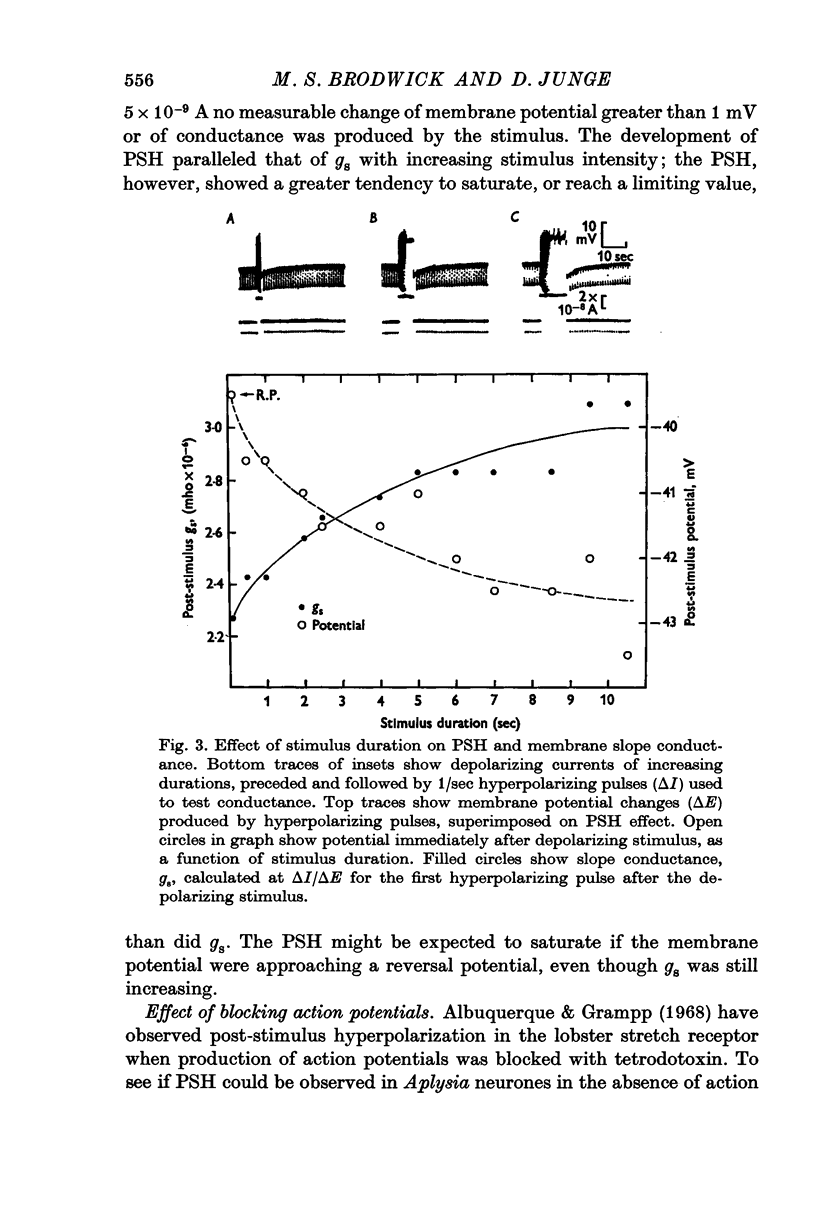
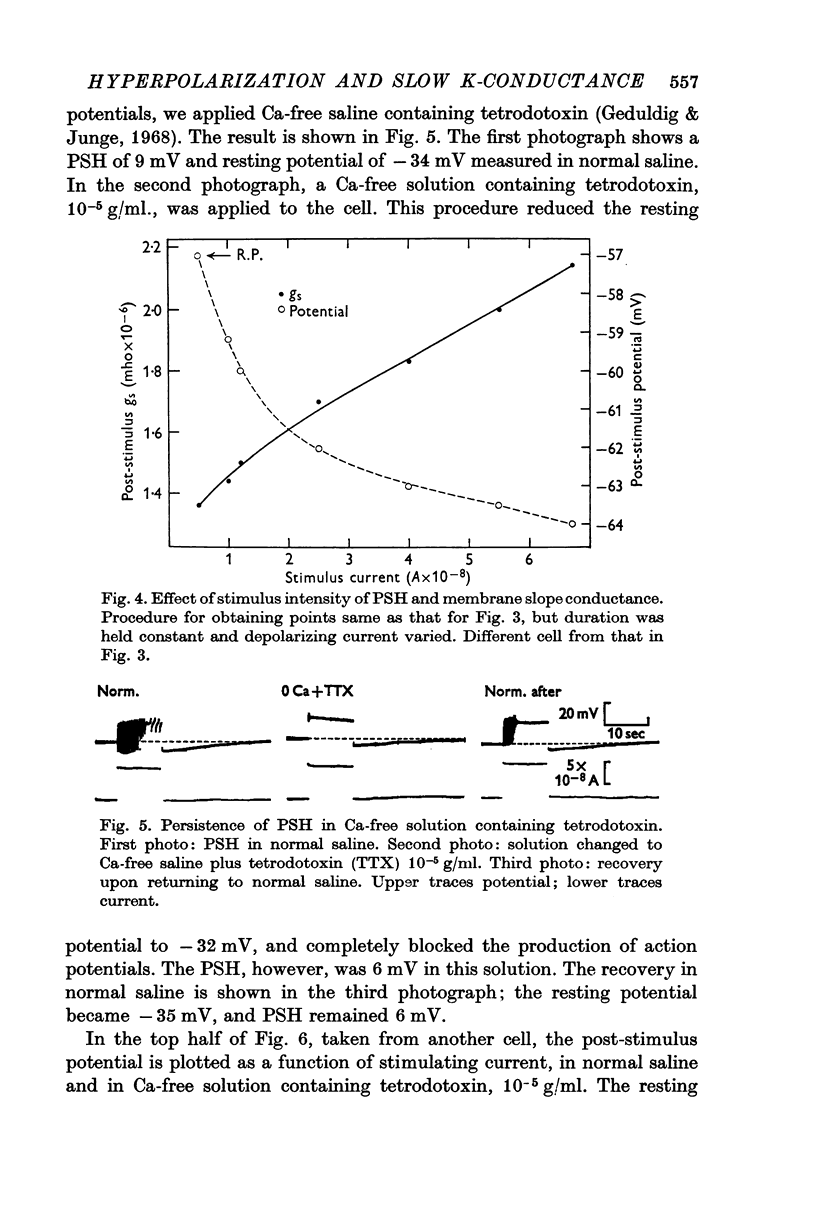
3. Both the PSH and conductance-increase varied strongly with stimulus amplitude and duration.
4. Both the PSH and the conductance increase occurred in Ca-free medium containing tetrodotoxin, when action-potential production was completely blocked.
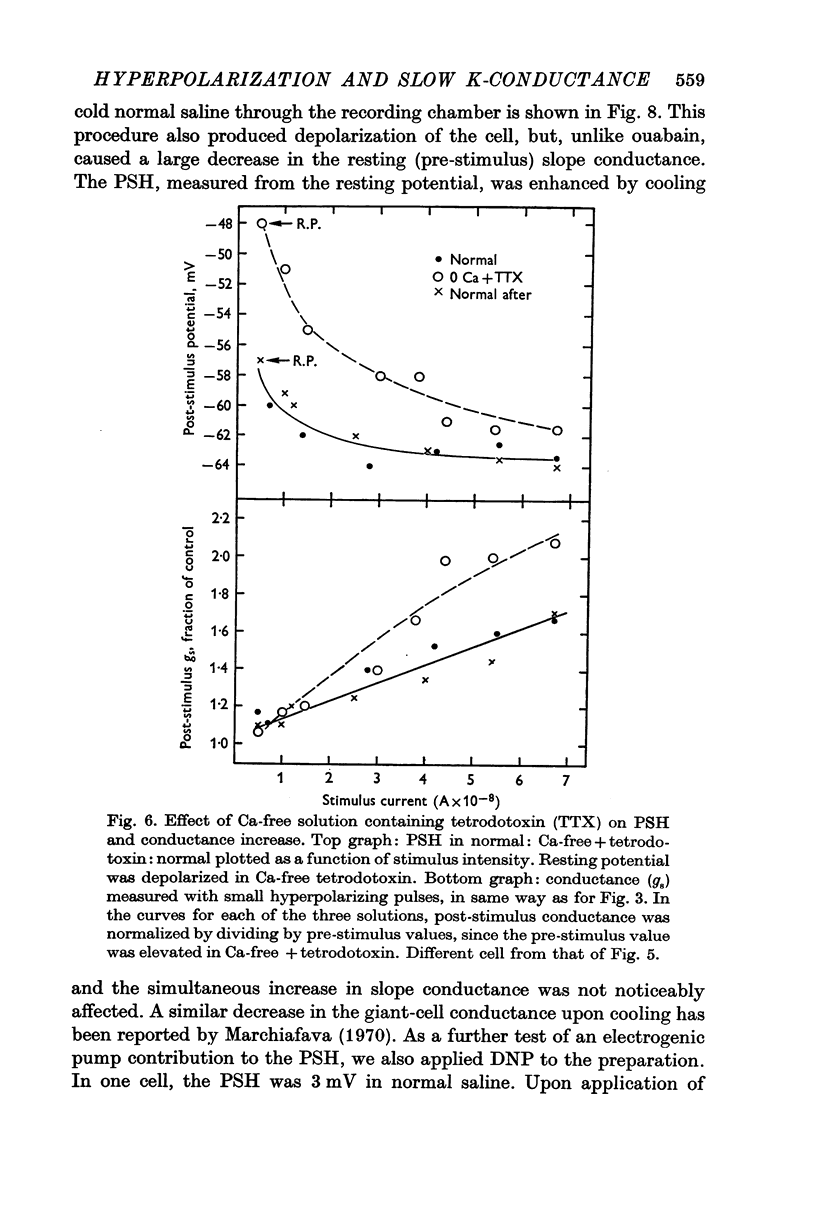
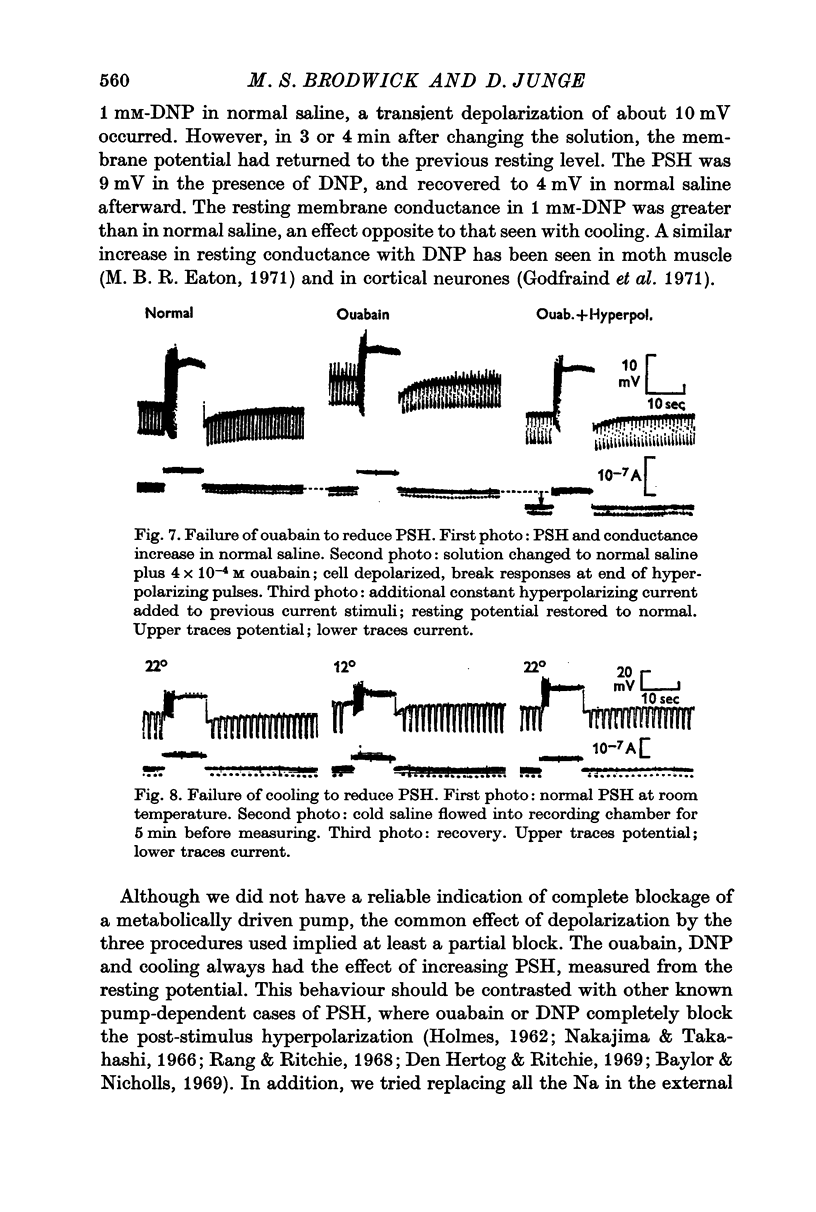
5. The PSH persisted in the presence of ouabain or DNP, with cooling, with removal of external K+, and in media where all the Na+ was replaced with Li+, suggesting that it was not due to the activity of an electrogenic pump.
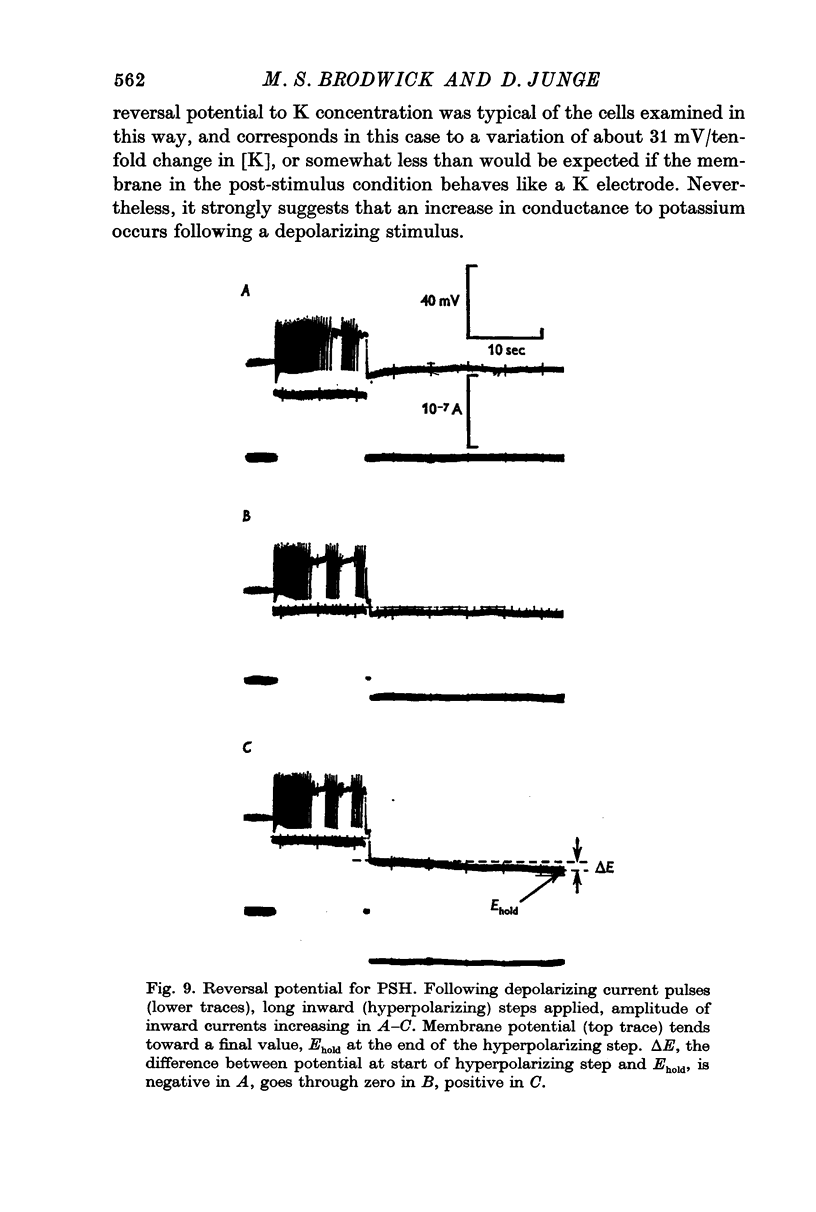
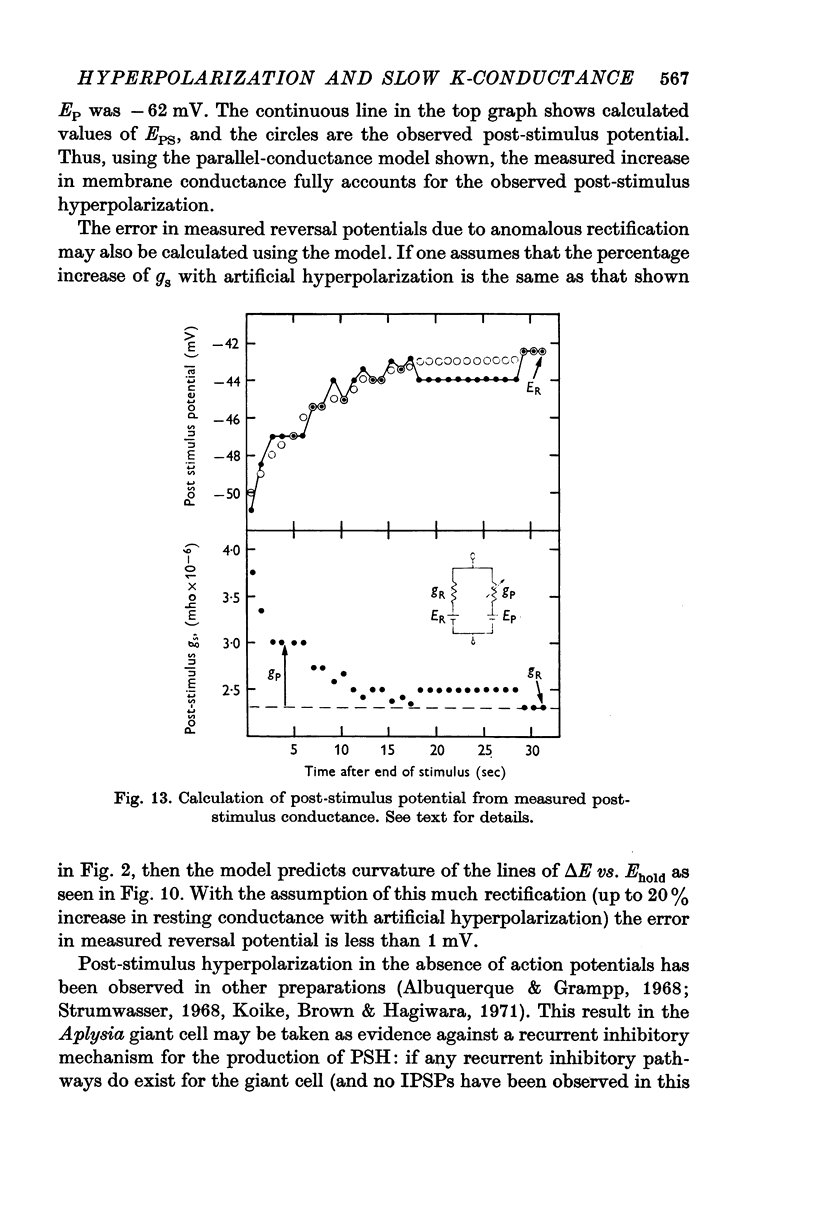
6. A reversal potential for the PSH was demonstrated by application of maintained inward current following the end of an outward-directed stimulus.
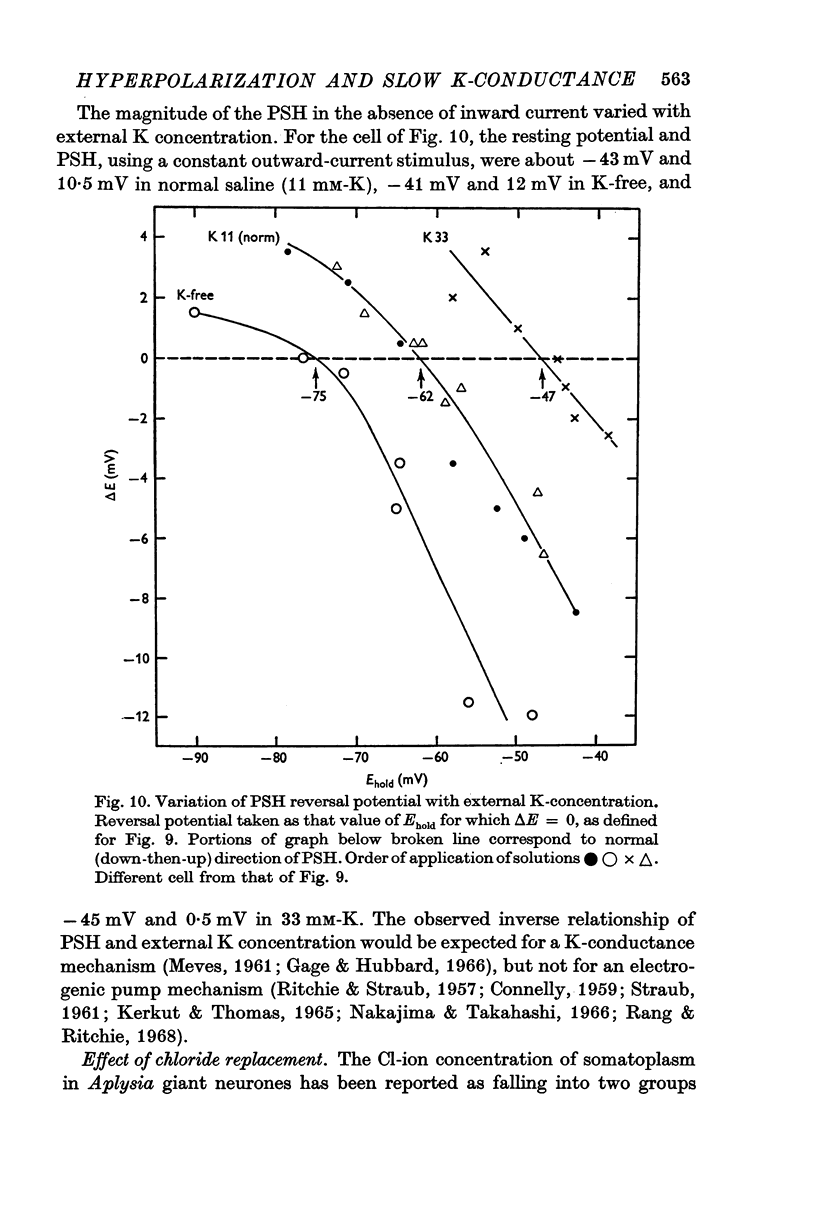
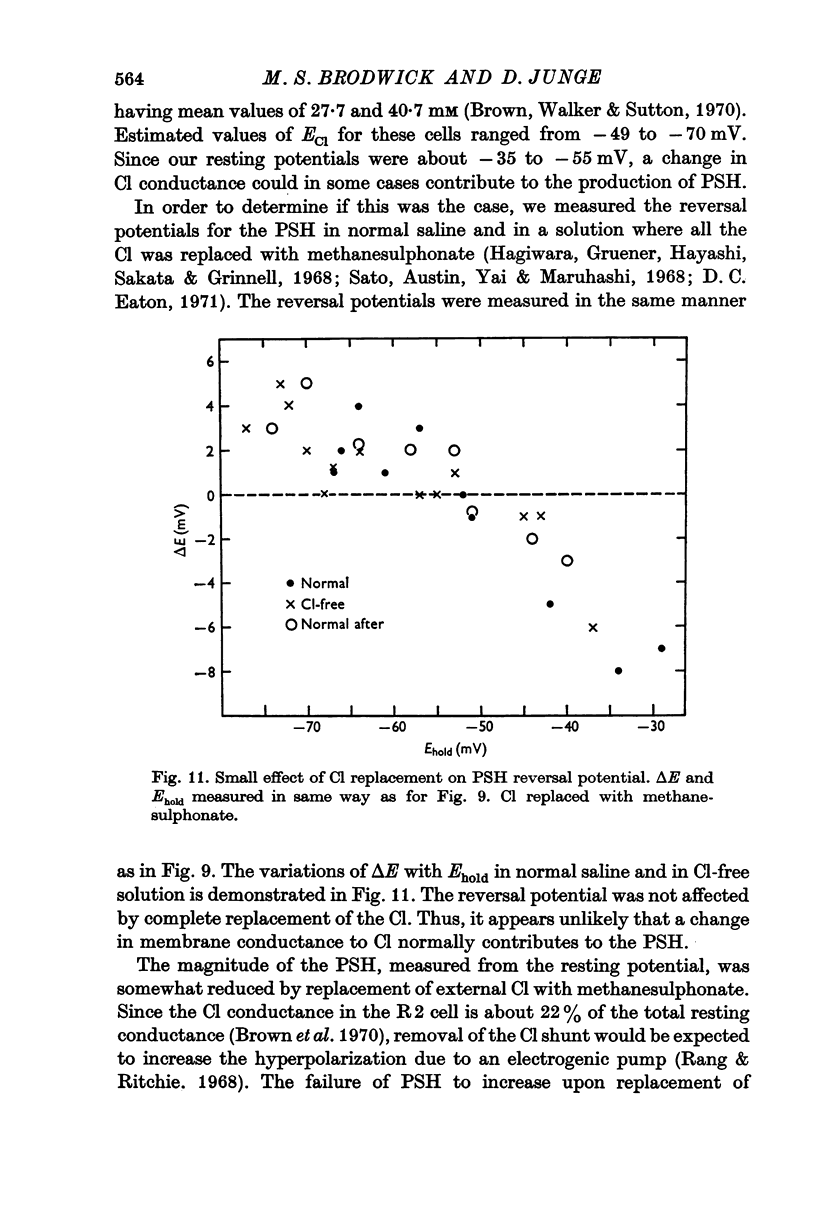
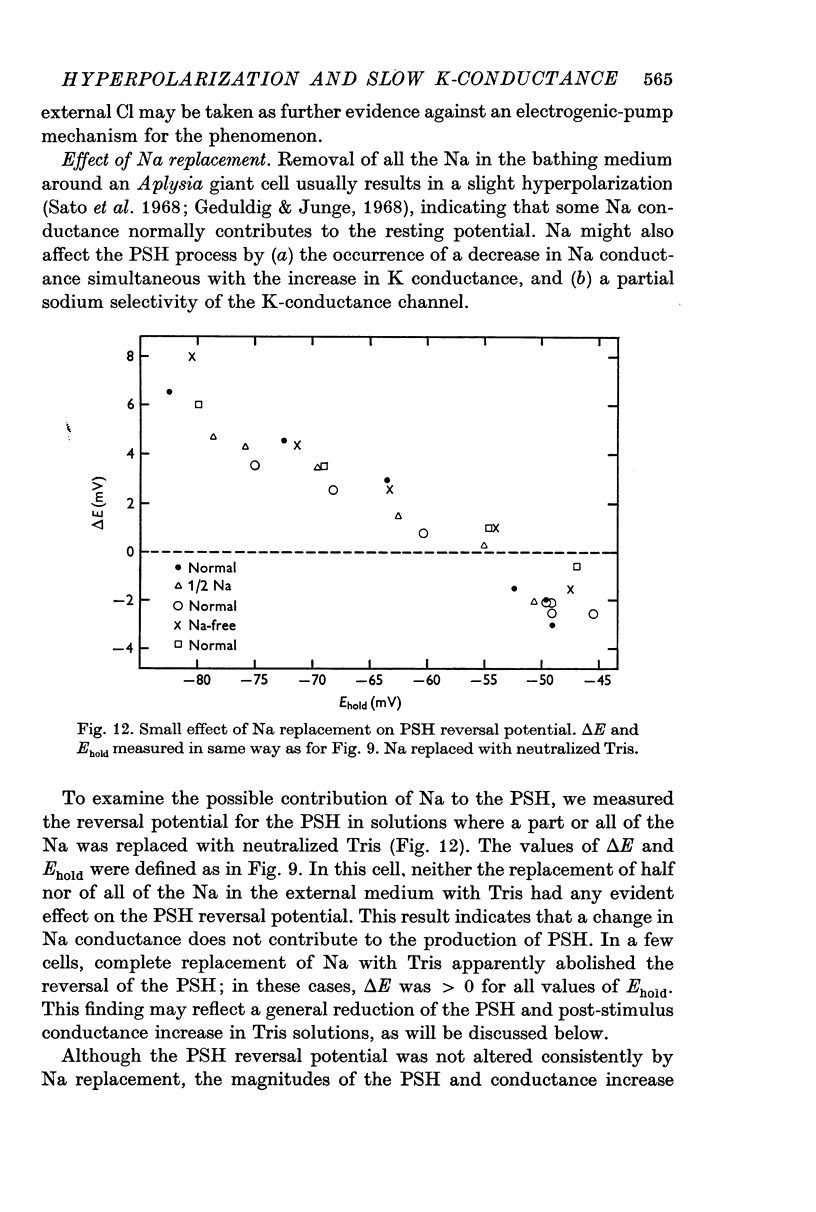
7. The PSH reversal potential varied with [K]o, but not with [Cl]o or [Na]o, suggesting that the PSH was mainly due to an increase in K conductance.
8. The PSH and the conductance increase were reduced strongly when all the Na+ was replaced with Tris, and only slightly when Na+ was replaced with sucrose.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Grampp W. Effects of tetrodotoxin on the slowly adapting stretch receptor neurone of lobster. J Physiol. 1968 Mar;195(1):141–156. doi: 10.1113/jphysiol.1968.sp008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):571–589. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettienne E. M. Control of contractility in Spirostomum by dissociated calcium ions. J Gen Physiol. 1970 Aug;56(2):168–179. doi: 10.1085/jgp.56.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Potassium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1962 Jan;160:54–61. doi: 10.1113/jphysiol.1962.sp006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind J. M., Kawamura H., Krnjević K., Pumain R. Actions of dinitrophenol and some other metabolic inhibitors on cortical neurones. J Physiol. 1971 May;215(1):199–222. doi: 10.1113/jphysiol.1971.sp009465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HOLMES O. Effects of pH, changes in potassium concentration and metabolic inhibitors on the after-potentials of mammalian non-medullated nerve fibres. Arch Int Physiol Biochim. 1962 Mar;70:211–245. doi: 10.3109/13813456209092855. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Gruener R., Hayashi H., Sakata H., Grinnell A. D. Effect of external and internal pH changes on K and Cl conductances in the muscle fiber membrane of a giant barnacle. J Gen Physiol. 1968 Nov;52(5):773–792. doi: 10.1085/jgp.52.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- Ko K. C., Gimeno A. L., Berman D. A. Effects of buffers on developed tension, membrane potentials, and ATP levels of atria. Am J Physiol. 1969 Apr;216(4):853–859. doi: 10.1152/ajplegacy.1969.216.4.853. [DOI] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T., Weakly J. N. Post-tetanic hyperpolarization produced by an electrogenic pump in dorsal spinocerebellar tract neurones of the cat. J Physiol. 1970 Nov;210(4):839–855. doi: 10.1113/jphysiol.1970.sp009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo Marchiafava P. The effect of temperature change on membrane potential and conductance in Aplysia giant nerve cell. Comp Biochem Physiol. 1970 Jun 15;34(4):847–852. doi: 10.1016/0010-406x(70)91007-8. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., YAMASAKI T. Mechanism of the after-potential production in the giant axons of the cockroach. J Physiol. 1960 Apr;151:75–88. [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Potassium movement in relation to nerve activity. J Gen Physiol. 1951 Jul;34(6):795–807. doi: 10.1085/jgp.34.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Austin G., Yai H., Maruhashi J. The ionic permeability changes during acetylcholine-induced responses of Aplysia ganglion cells. J Gen Physiol. 1968 Mar;51(3):321–345. doi: 10.1085/jgp.51.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove P. G., Cooke I. M. Inhibition of impulse activity in a sensory neuron by an electrogenic pump. J Gen Physiol. 1971 Feb;57(2):125–163. doi: 10.1085/jgp.57.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., KANDEL E. R. AN ANOMALOUS FORM OF RECTIFICATION IN A MOLLUSCAN CENTRAL NEURONE. Nature. 1964 Jun 27;202:1339–1341. doi: 10.1038/2021339a0. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968 Jan;51(1):65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A., Ritchie J. M. A comparison of the effect of temperature, metabolic inhibitors and of ouabain on the electrogenic componen of the sodium pump in mammalian non-myelinated nerve fibres. J Physiol. 1969 Oct;204(3):523–538. doi: 10.1113/jphysiol.1969.sp008929. [DOI] [PMC free article] [PubMed] [Google Scholar]


