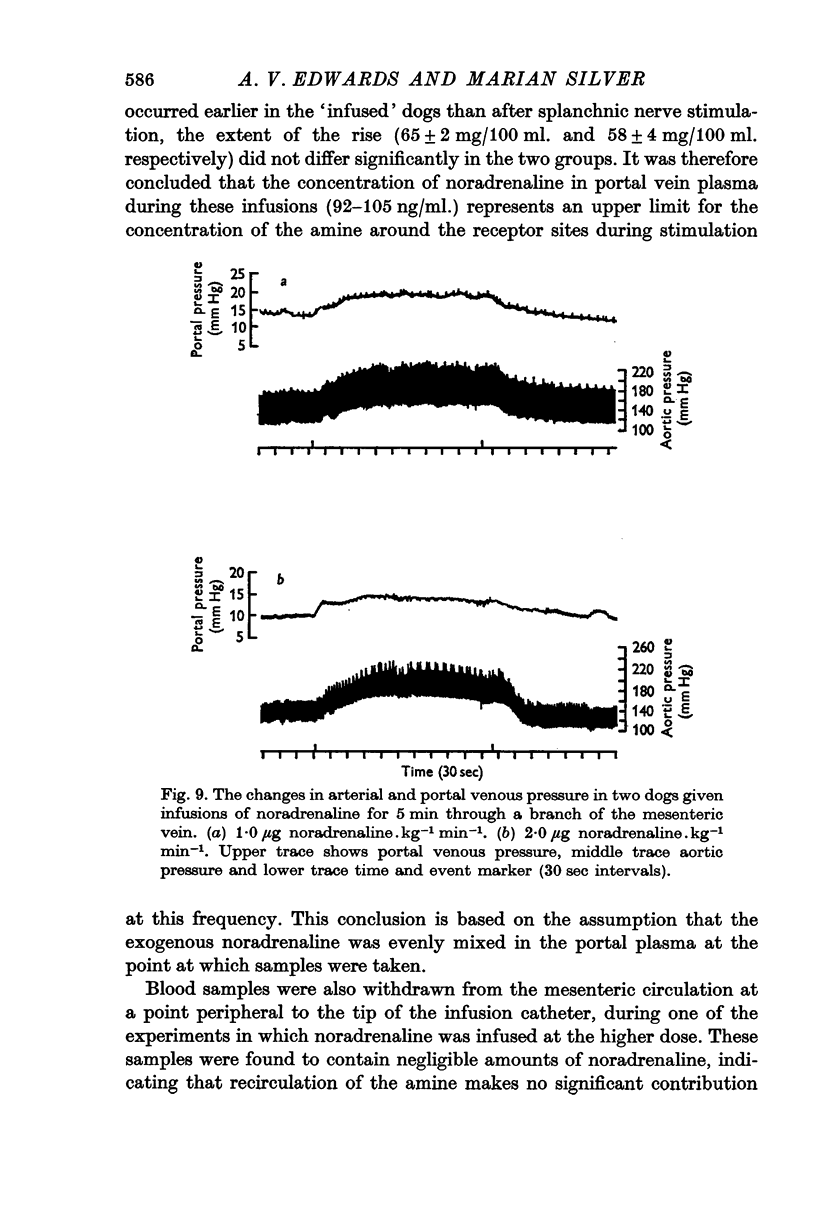
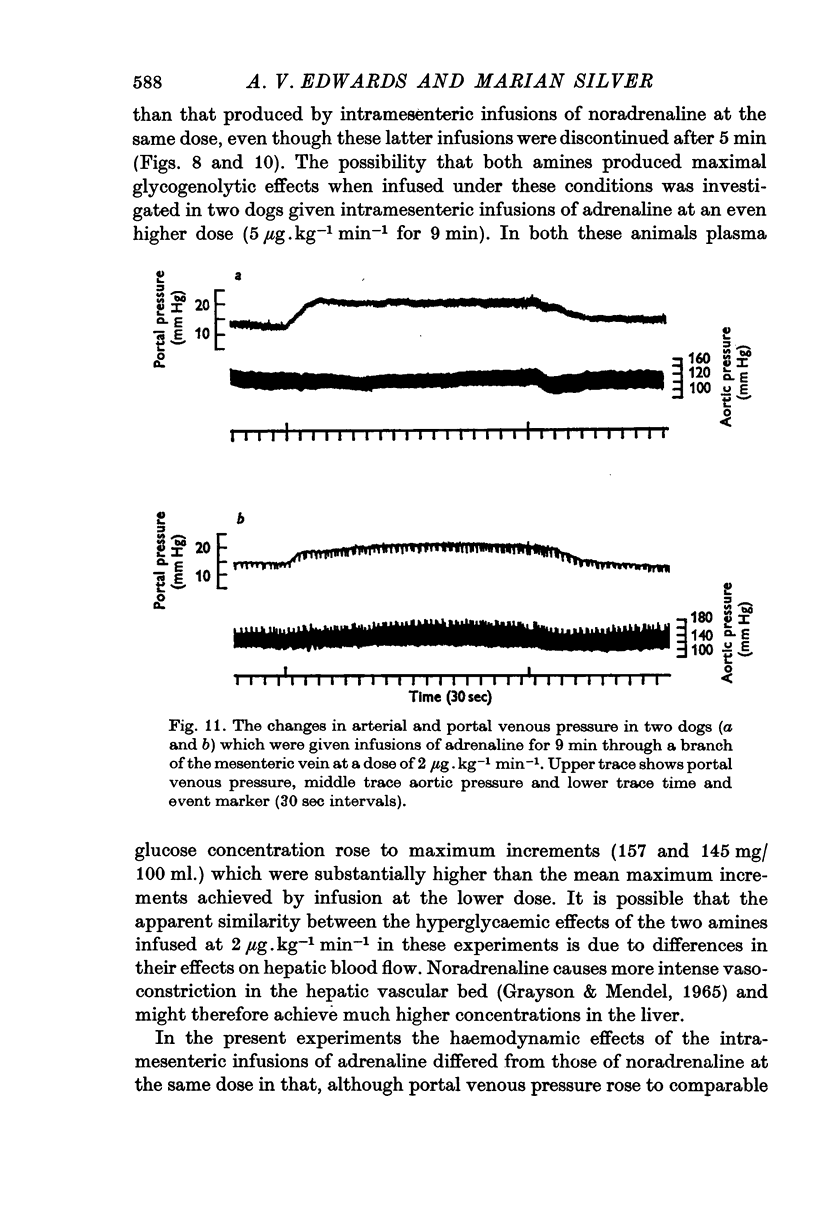
Abstract
1. The effects of stimulation of the splanchnic innervation to the adrenal medullae, in dogs with cut hepatic nerves, were compared with those obtained previously in response to splanchnic and hepatic nerve stimulation in adrenalectomized dogs.
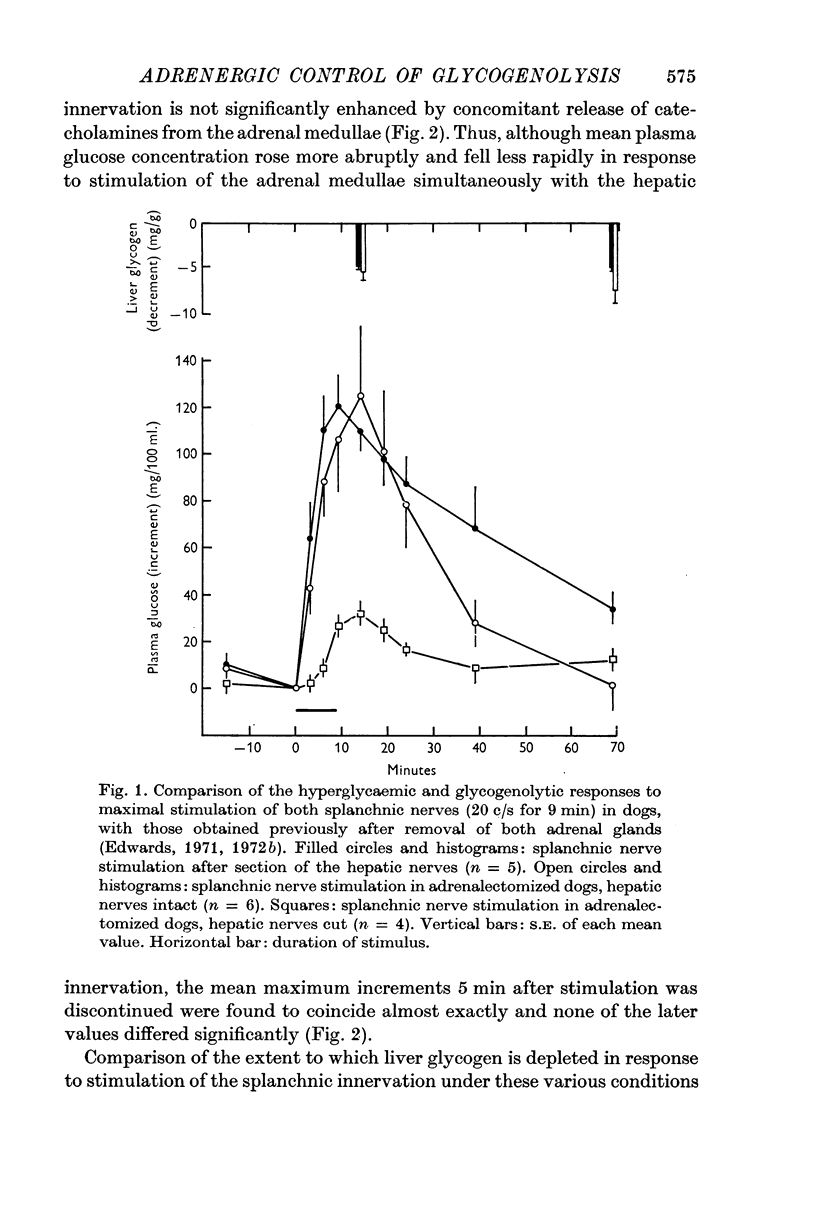
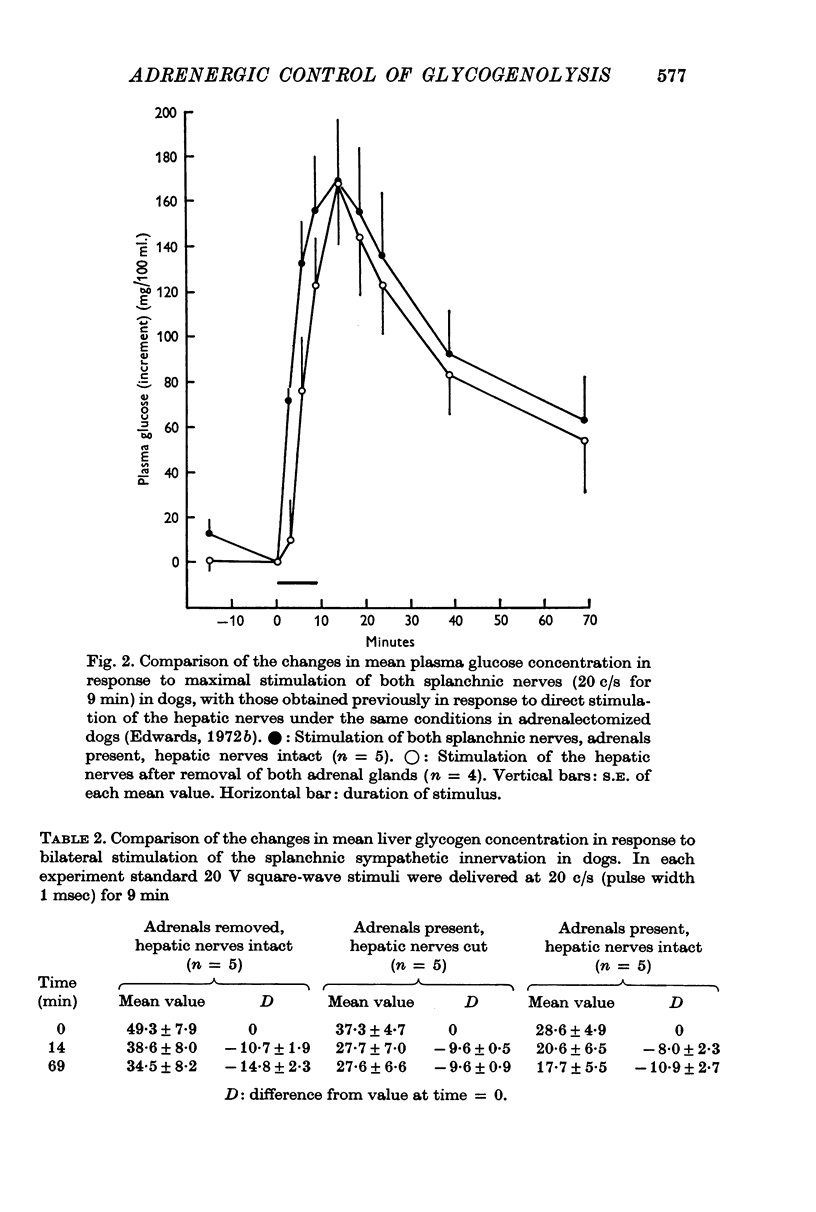
2. Maximal stimulation of both adrenal medullae via the splanchnic innervation (20 c/s for 9 min), in dogs with cut hepatic nerves, produced closely similar hyperglycaemic and glycogenolytic responses to those obtained previously in adrenalectomized dogs with intact hepatic nerves.
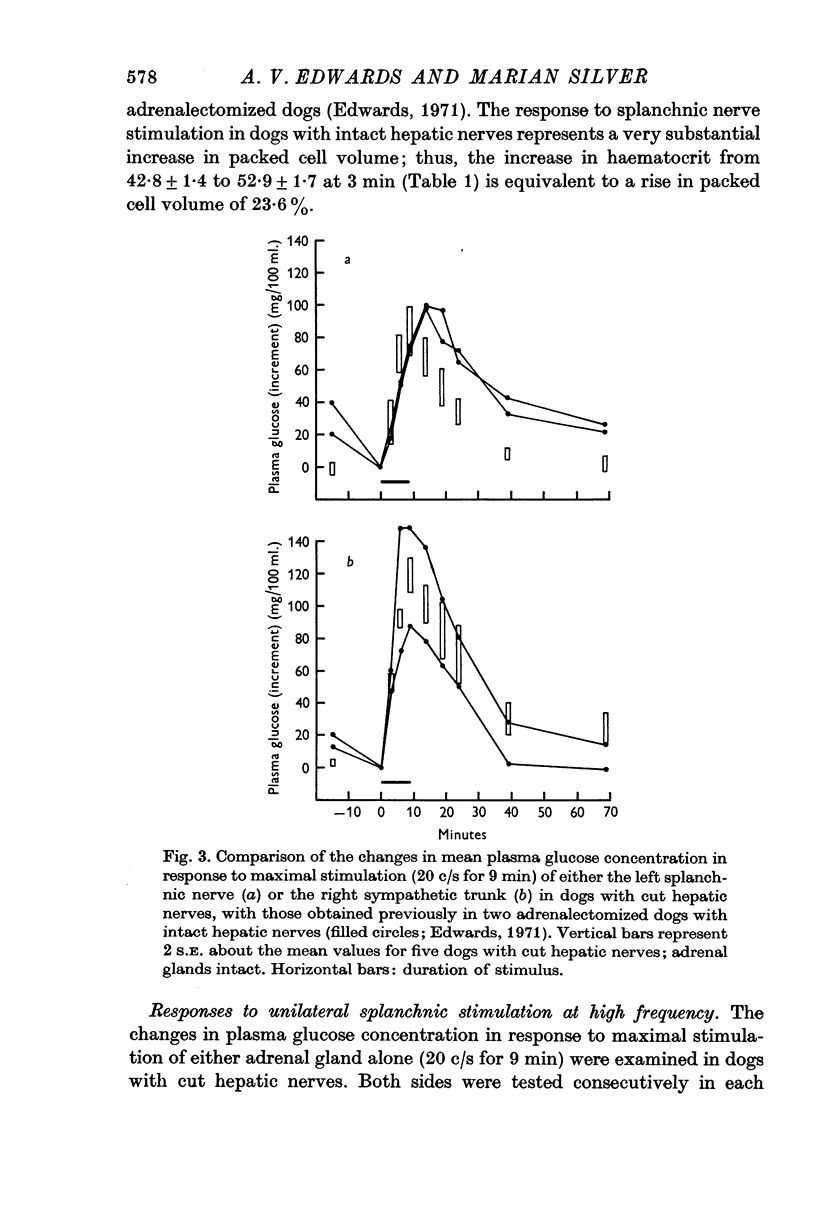
3. The rise in plasma glucose concentration in response to maximal stimulation of the adrenal medullae in dogs with intact hepatic nerves was found to be comparable to that which occurs in response to maximal stimulation of the hepatic sympathetic innervation alone. In contrast, the rise in haematocrit during maximal stimulation of the entire splanchnic innervation was substantially greater than that observed after removal of both adrenal glands.
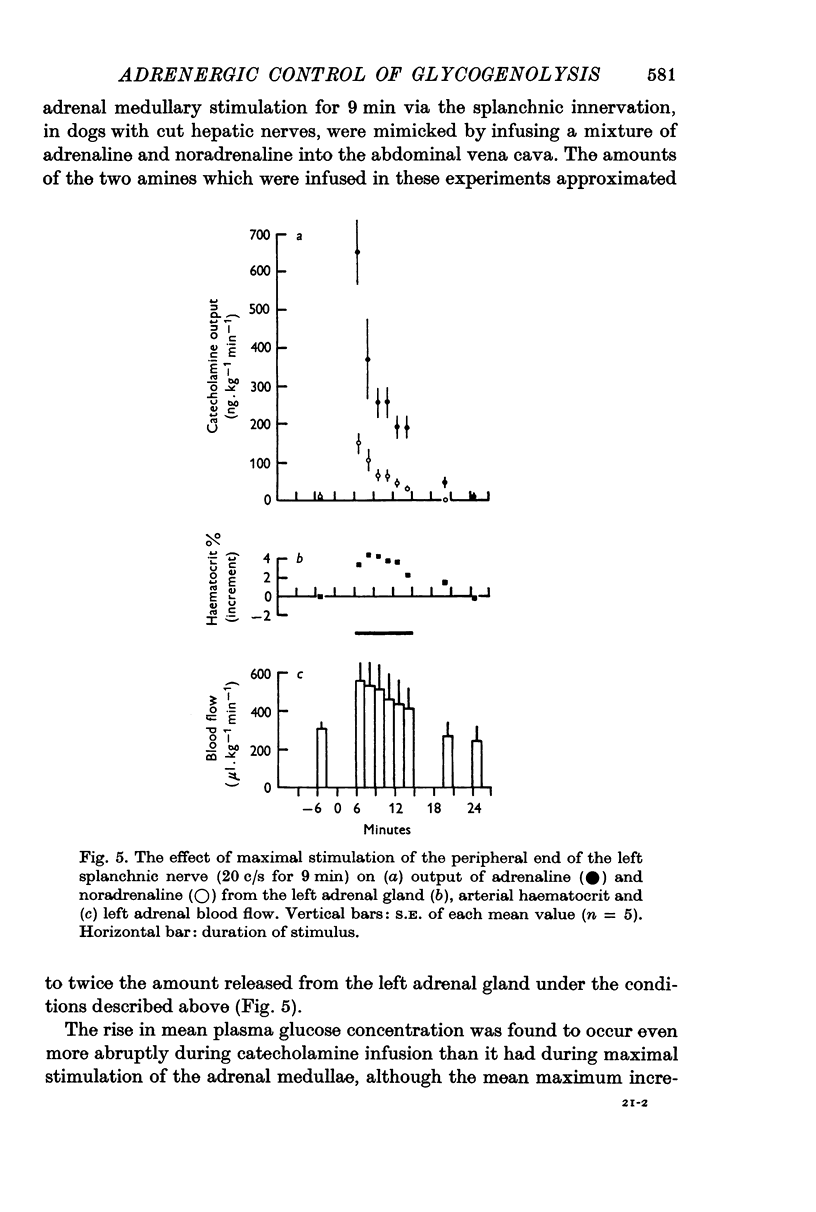
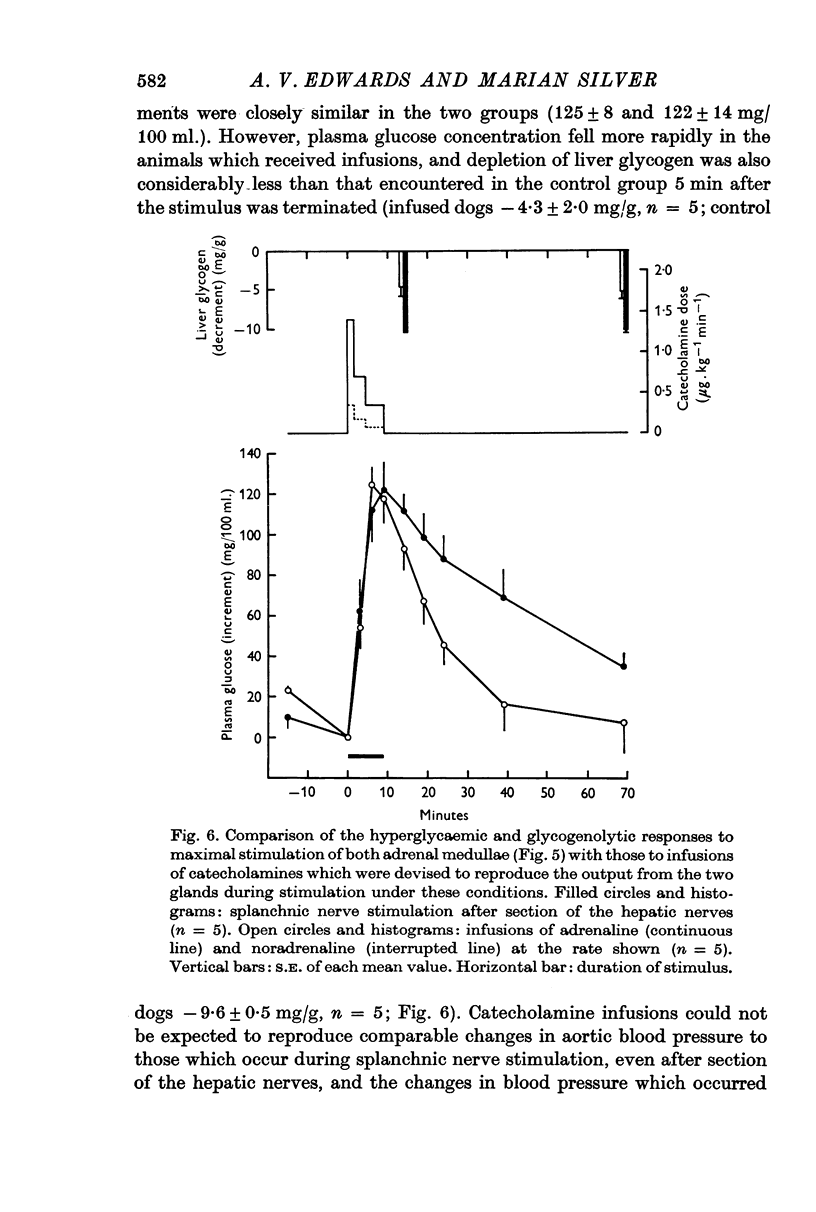
4. The output of adrenaline and noradrenaline from the left adrenal gland was determined during maximal stimulation of the left splanchnic nerve (20 c/s for 9 min). These results were then used to compute doses of the two amines which would reproduce the output of catecholamines from both glands under such conditions. The extent of the rise in mean plasma glucose concentration in response to these infusions was similar to that produced by maximal stimulation of both adrenal glands, but the duration of hyperglycaemia and depletion of liver glycogen were significantly less.
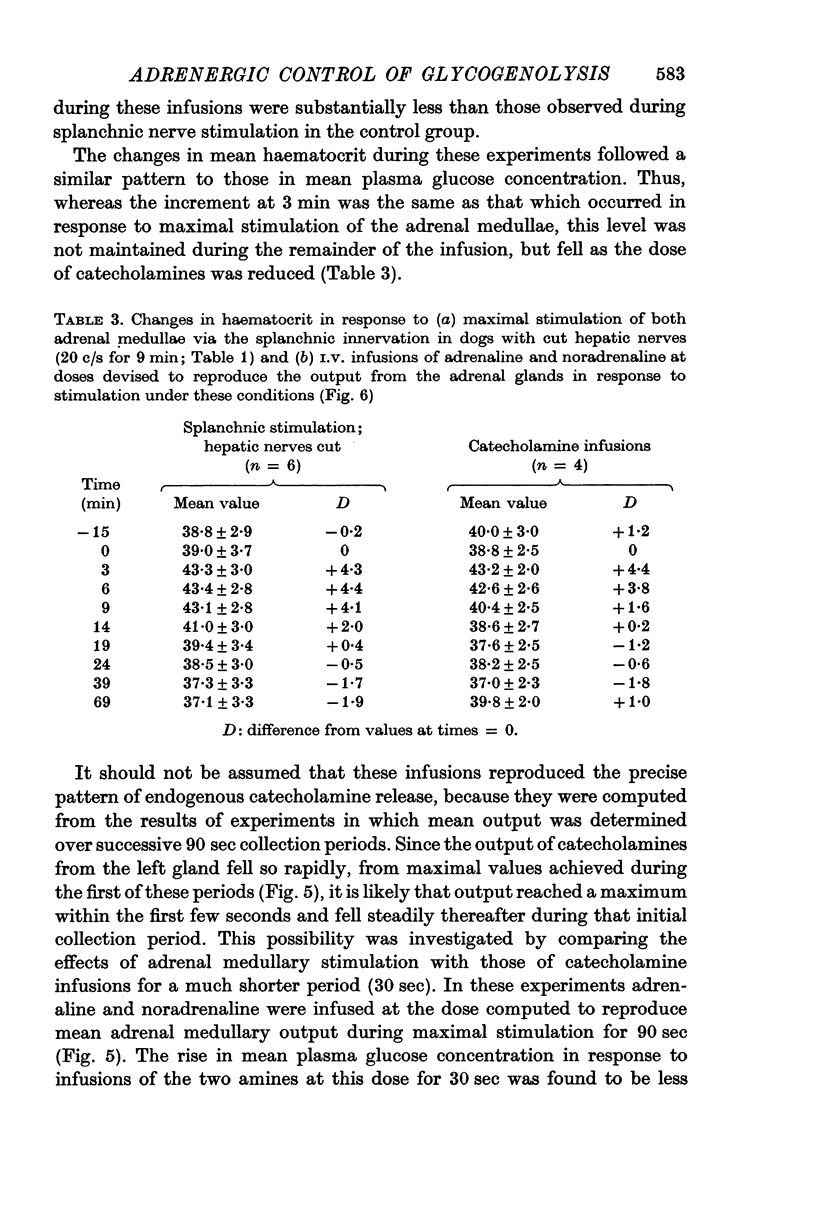
5. Stimulation of the splanchnic innervation was found to produce an initial `surge' in the release of catecholamines from the adrenal medullae, followed by a rapid decline in output when stimulation was continued for longer than 30 sec. Evidence was obtained which showed that this pattern of release is well suited to produce rapid mobilization of liver glycogen.
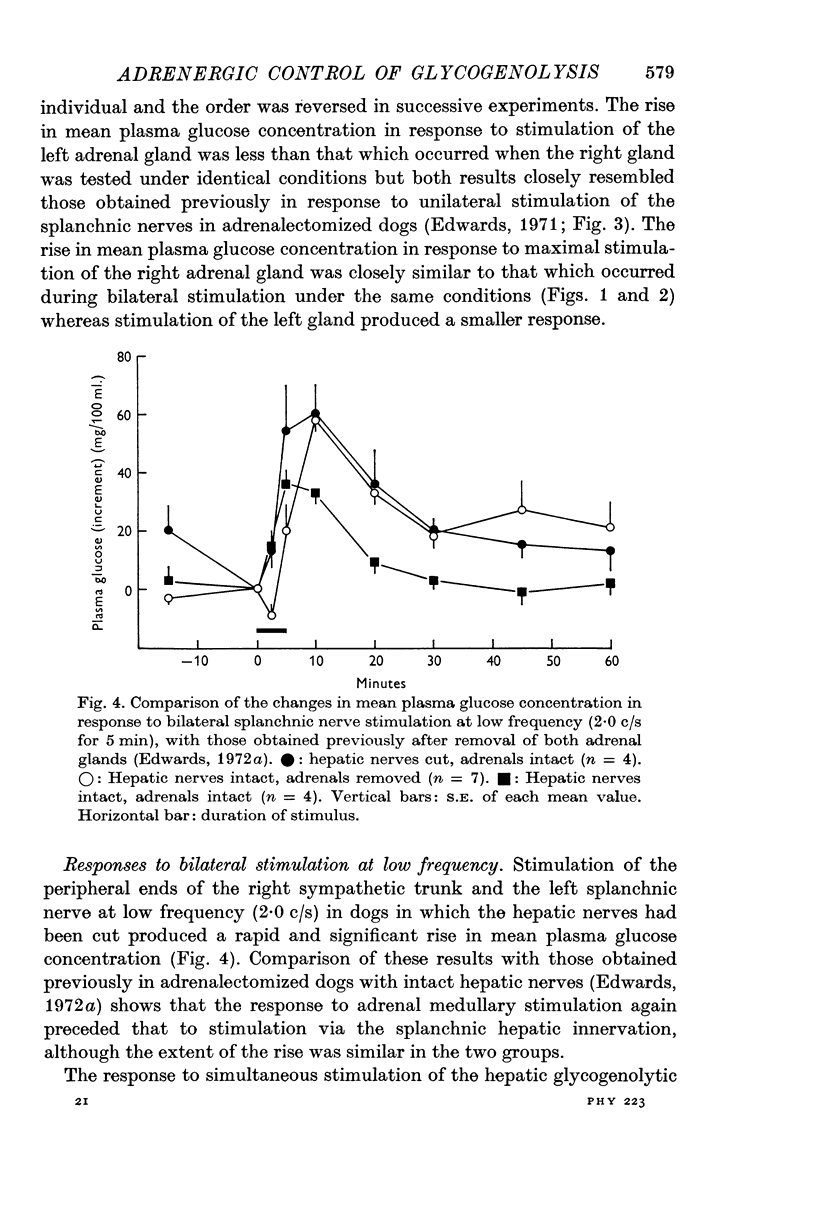
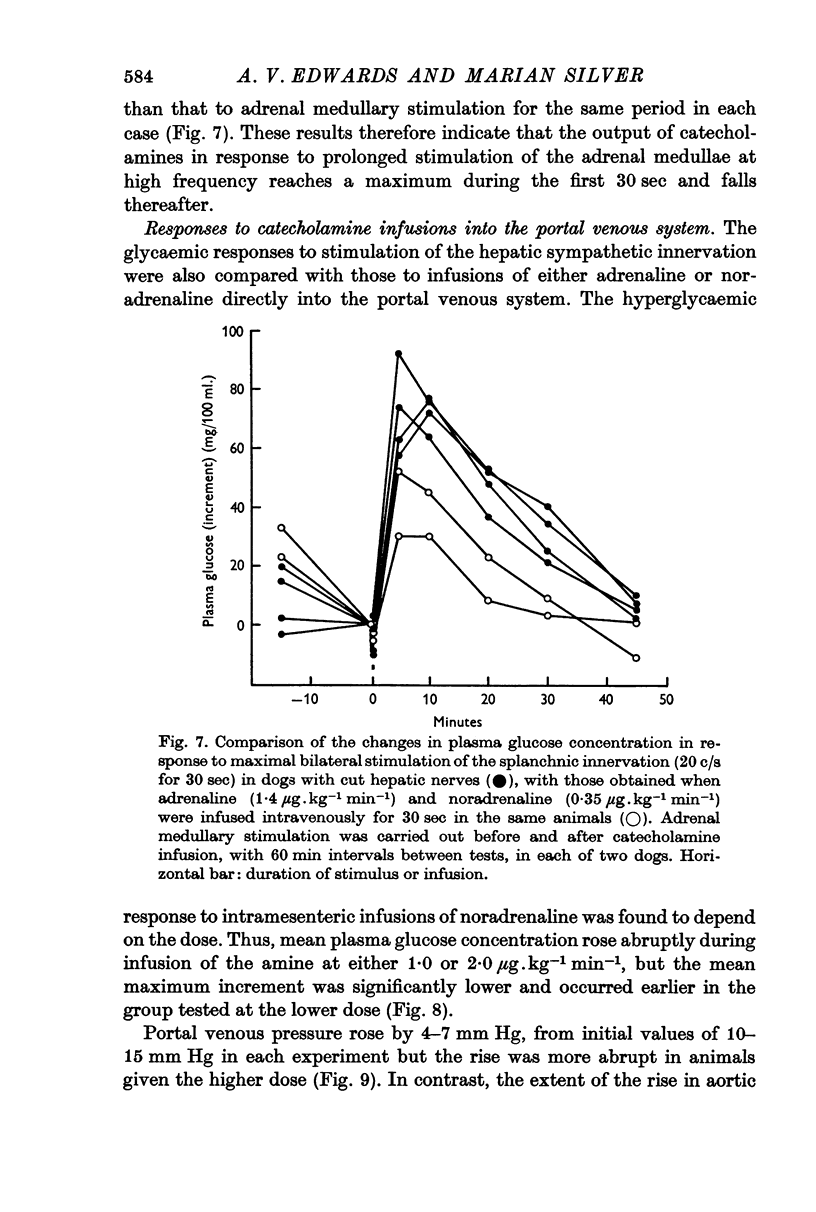
6. Comparable changes in plasma glucose concentration occurred in response to stimulation of either the adrenal medullae or the sympathetic innervation to the liver at low frequency (2·0 c/s for 5 min). Stimulation of both pathways simultaneously, at the same frequency, produced smaller responses.
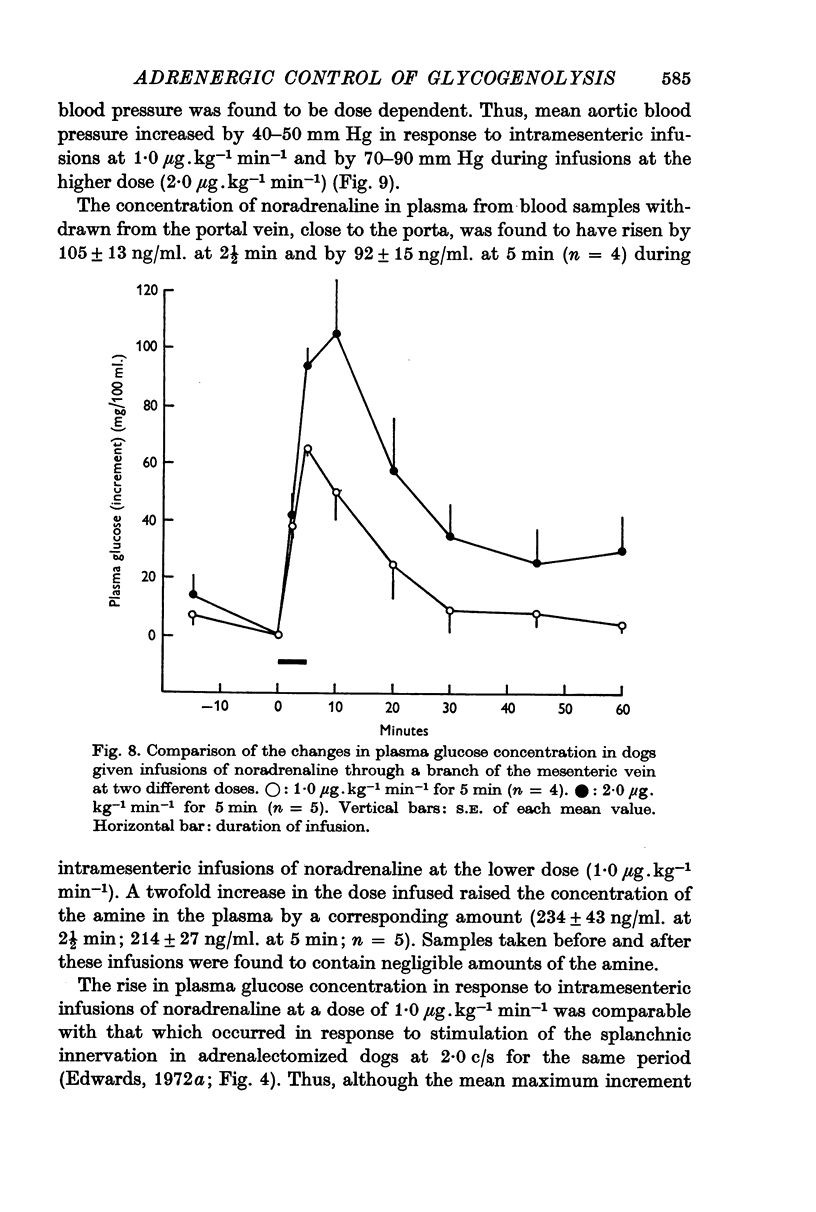
7. Intramesenteric infusions of noradrenaline at 1·0 μg.kg-1 min-1 for 5 min produced comparable changes in plasma glucose concentration to those observed during stimulation of either pathway alone at low frequency. The mean plasma noradrenaline concentration of portal blood was raised by between 92 and 105 ng/ml. during these infusions.
8. It is concluded that stimulation of either the splanchnic innervation to the liver, or of both adrenal medullae, at high frequency (20 c/s for 9 min) represents a supramaximal stimulus for hepatic glycogenolysis. Comparison of the responses to stimulation at low frequency (2·0 c/s for 5 min) suggests that the hepatic glycogenolytic mechanism is equally sensitive to stimulation via either route in this species.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T. Adrenaline release during insulin hypoglycaemia in the rabbit. J Physiol. 1959 Dec;149:228–249. doi: 10.1113/jphysiol.1959.sp006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Reis D. J. Central neural regulation by adrenergic nerves of the daily rhythm in hepatic tyrosine transaminase activity. J Physiol. 1971 Dec;219(2):267–280. doi: 10.1113/jphysiol.1971.sp009661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Reis D. J. Cholinergic regulation of hepatic tyrosine transaminase activity. J Physiol. 1971 Mar;213(2):421–433. doi: 10.1113/jphysiol.1971.sp009391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Silver M. The glycogenolytic response to stimulation of the splanchnic nerves in adrenalectomized calves. J Physiol. 1970 Nov;211(1):109–124. doi: 10.1113/jphysiol.1970.sp009269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. The glycogenolytic response to stimulation of the splanchnic nerves in adrenalectomized calves, sheep, dogs, cats and pigs. J Physiol. 1971 Mar;213(3):741–759. doi: 10.1113/jphysiol.1971.sp009412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. The hyperglycaemic response to stimulation of the hepatic sympathetic innervation in adrenalectomized cats and dogs. J Physiol. 1972 Feb;220(3):697–710. doi: 10.1113/jphysiol.1972.sp009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. The sensitivity of the hepatic glycogenolytic mechanism ot stimulation of the splanchnic nerves. J Physiol. 1972 Jan;220(2):315–334. doi: 10.1113/jphysiol.1972.sp009709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFIEN A., ZILELI M. S., DESPOINTES R. H., BETHUNE J. E. The effects of hypoglycemia on the adrenal secretion of epinephrine and norepinephrine in the dog. Endocrinology. 1958 Jun;62(6):749–757. doi: 10.1210/endo-62-6-749. [DOI] [PubMed] [Google Scholar]
- GREEN H. D., OTTIS K., KITCHEN T. Autonomic stimulation and blockade on canine splenic inflow, outflow and weight. Am J Physiol. 1960 Feb;198:424–428. doi: 10.1152/ajplegacy.1960.198.2.424. [DOI] [PubMed] [Google Scholar]
- Greenway C. V., Lautt W. W., Stark R. D. Capacitance responses and fluid exchange in the cat liver. J Physiol. 1969 Nov;205(2):33P–34P. [PubMed] [Google Scholar]
- Greenway C. V., Lawson A. E., Mellander S. The effects of stimulation of the hepatic nerves, infusions of noradrenaline and occlusion of the carotid arteries on liver blood flow in the anaesthetized cat. J Physiol. 1967 Sep;192(1):21–41. doi: 10.1113/jphysiol.1967.sp008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway C. V., Lawson A. E. The effects of adrenaline and noradrenaline on venous return and regional blood flows in the anaesthetized cat with special reference to intestinal blood flow. J Physiol. 1966 Oct;186(3):579–595. doi: 10.1113/jphysiol.1966.sp008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- KRAMER K., LUFT U. C. Mobilization of red cells and oxygen from the spleen in severe hypoxia. Am J Physiol. 1951 Apr 1;165(1):215–228. doi: 10.1152/ajplegacy.1951.165.1.215. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Amakawa A. Regulation of glycogen metabolism in liver by the autonomic nervous system. II. Neural control of glycogenolytic enzymes. Biochim Biophys Acta. 1968 Oct 15;165(3):335–348. doi: 10.1016/0304-4165(68)90211-0. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Fujimoto T. Regulation of glycogen metabolism in liver by the autonomic nervous system. IV. Neural control of glycogen biosynthesis. Biochim Biophys Acta. 1971 Oct;252(1):18–27. doi: 10.1016/0304-4165(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Regulation of glycogen metabolism in liver by the autonomic nervous system. V. Activation of glycogen synthetase by vagal stimulation. Biochim Biophys Acta. 1971 Oct;252(1):28–38. doi: 10.1016/0304-4165(71)90089-4. [DOI] [PubMed] [Google Scholar]


