Abstract
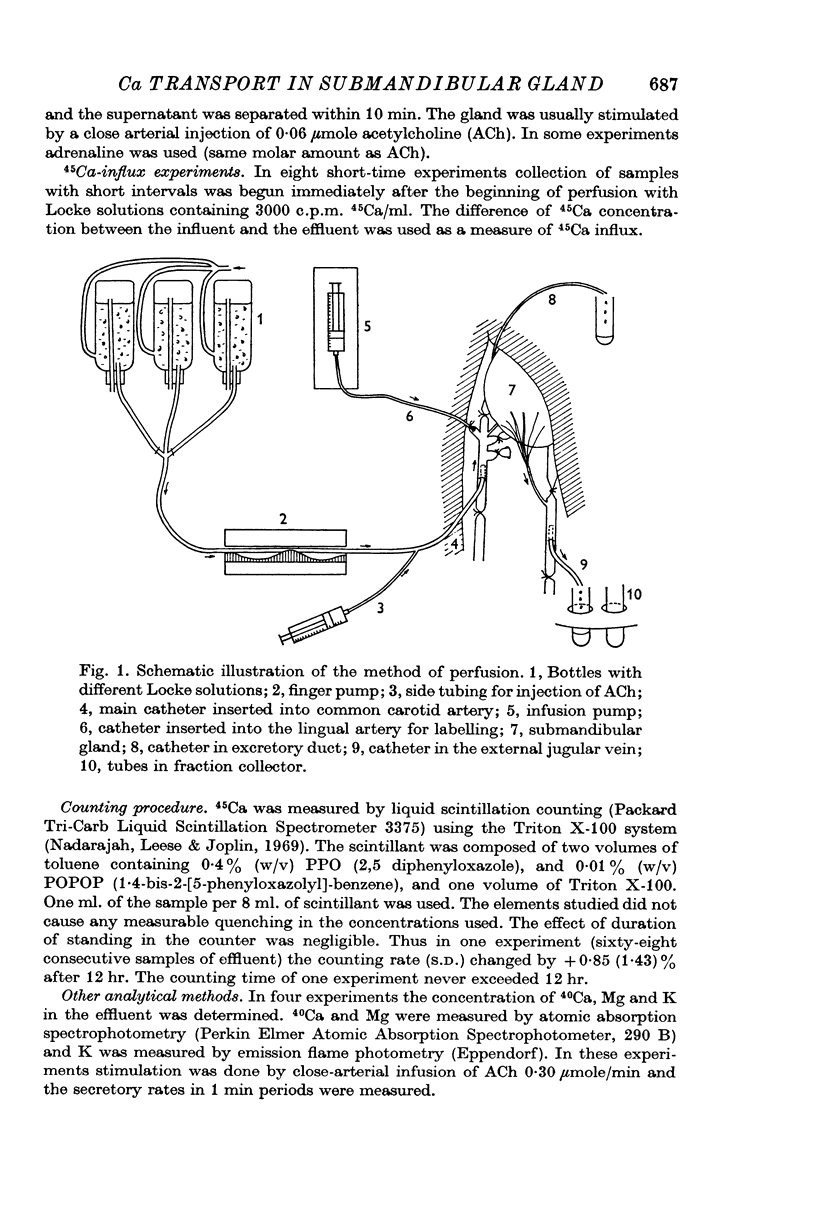
1. In the perfused cat submandibular gland efflux and influx of 45Ca, and concentrations of K, 40Ca and Mg in the effluent from the gland were measured under different experimental conditions.
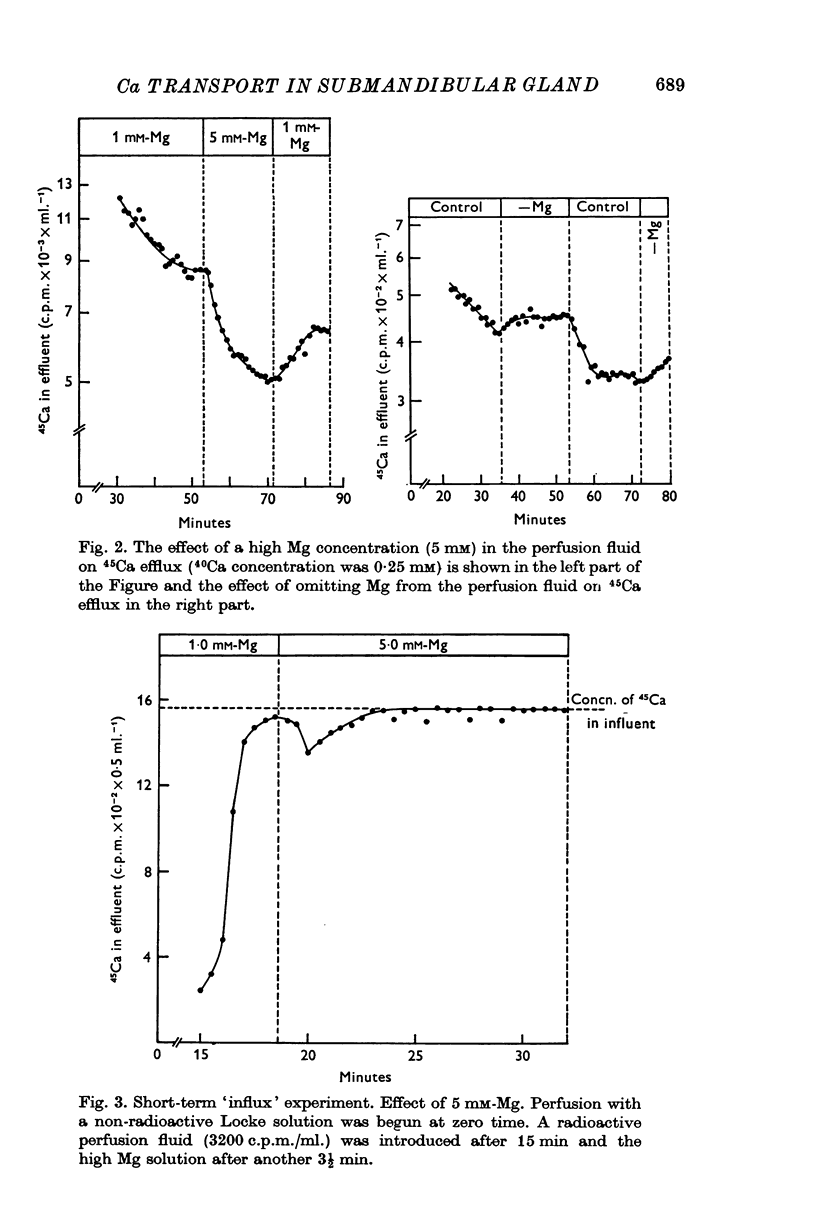
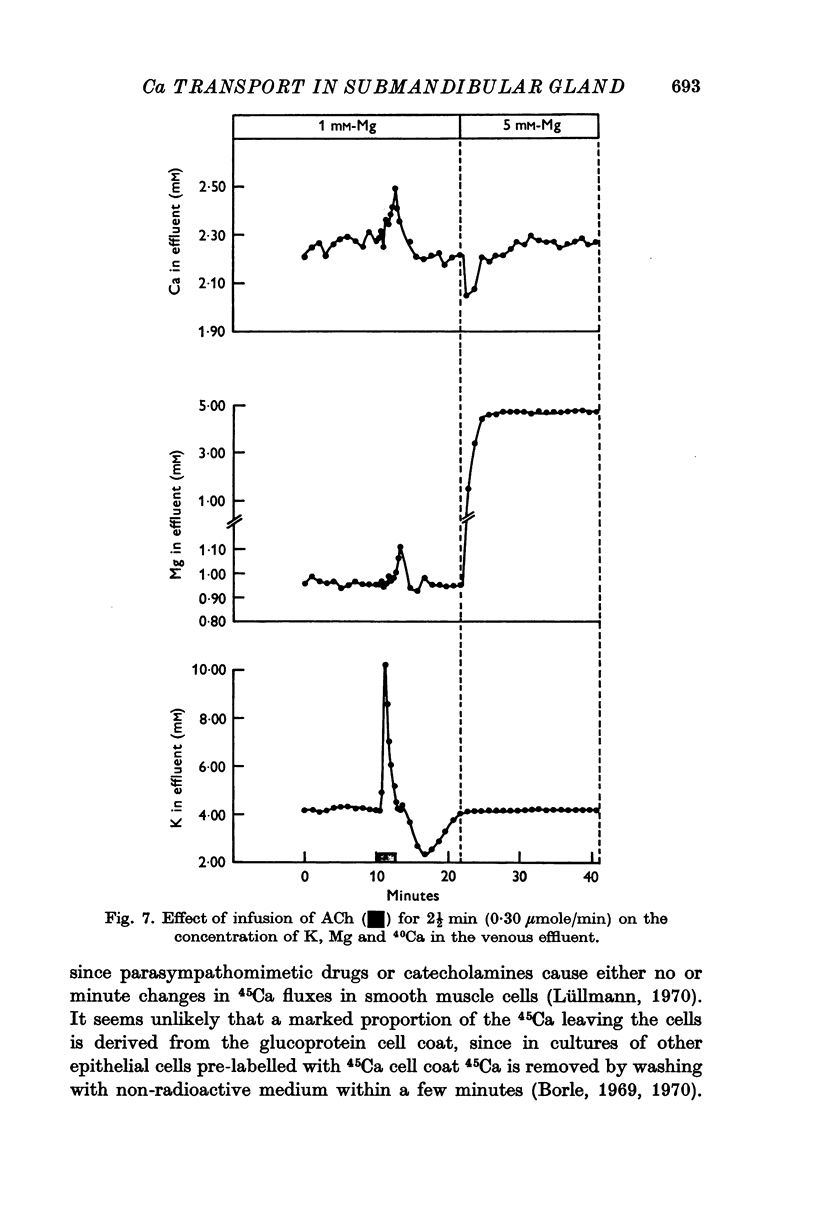
2. When the standard perfusion fluid was shifted to a high Mg (5 mM) or a low Ca (0·25 mM) solution the efflux of 45Ca from the pre-labelled gland declined. The magnitude and the duration of the effect of the high Mg concentration was more marked at a low external Ca concentration and was abolished by Mersalyl (1 mM). When the standard perfusion fluid was shifted to a Mg-free solution the efflux of 45Ca from the pre-labelled gland increased.
3. After shift of 45Ca containing perfusion fluid from normal to a high Mg (5 mM) solution the influx of 45Ca to the gland increased rapidly.
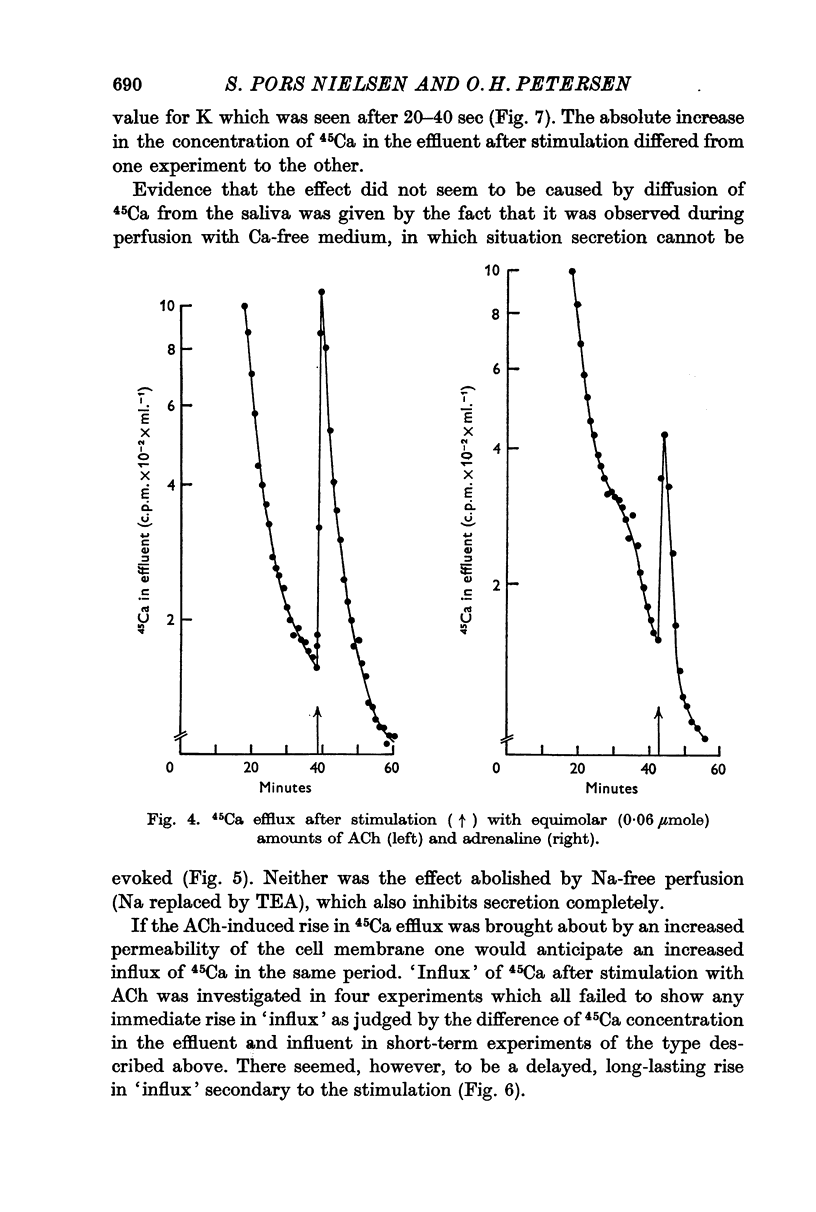
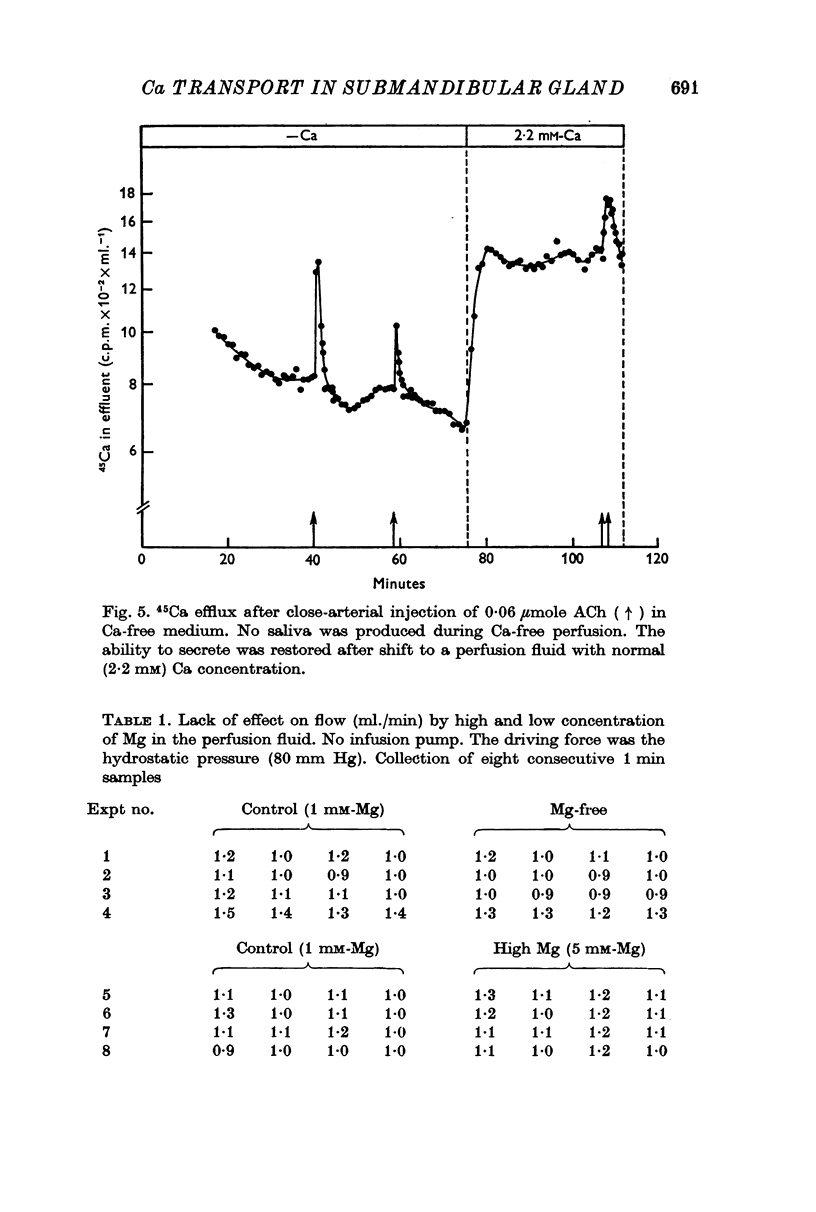
4. Both acetylcholine (ACh) and adrenaline caused a marked increase in the efflux of 45Ca from the pre-labelled gland. This increase in efflux was also seen under conditions where the gland was unable to secrete, i.e. during perfusion with Ca-free and Na-free tetraethylammonium Locke solutions.
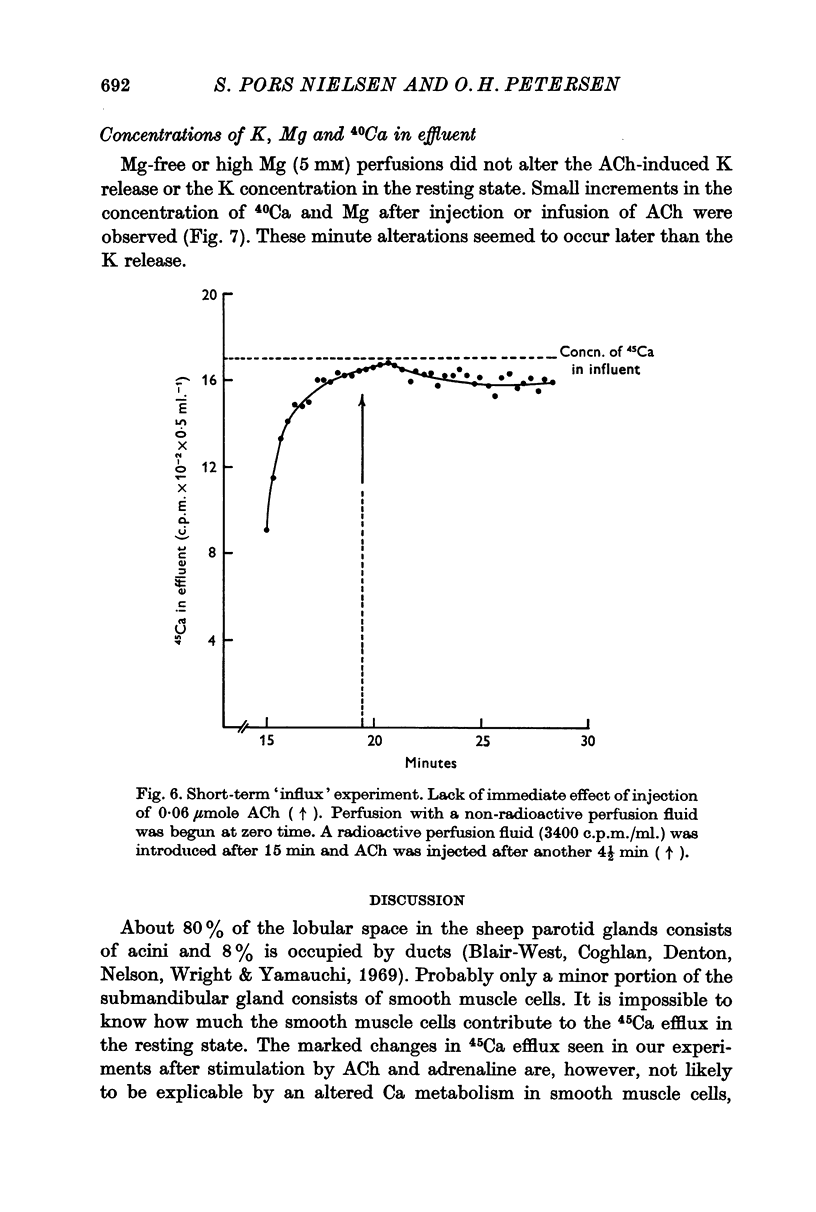
5. Stimulation with ACh failed to reveal any rapidly occurring increase in influx of 45Ca.
6. Stimulation with ACh evoked a small temporary increase in the concentration of 40Ca. and Mg in the effluent.
7. It is suggested that Ca uptake by intracellular Ca-accumulating systems of the submandibular gland depends on the external Mg concentration and that ACh and adrenaline cause a release of Ca bound intracellularly.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. The secretion of potassium in saliva. J Physiol. 1956 Apr 27;132(1):20–39. doi: 10.1113/jphysiol.1956.sp005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-West J. R., Coghlan J. P., Denton D. A., Nelson J., Wright R. D., Yamauchi A. Ionic, histological and vascular factors in the reaction of the sheep's parotid to high and low mineralocorticoid status. J Physiol. 1969 Dec;205(3):563–579. doi: 10.1113/jphysiol.1969.sp008983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. II. Calcium efflux. J Gen Physiol. 1969 Jan;53(1):57–69. doi: 10.1085/jgp.53.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in cell cultures. 3. Effects of calcium and parathyroid hormone in kidney cells. J Gen Physiol. 1970 Feb;55(2):163–186. doi: 10.1085/jgp.55.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREISBACH R. H. CALCIUM TRANSFER IN RAT SALIVARY AND LACRIMAL GLANDS. Int Ser Monogr Oral Biol. 1964;3:237–251. doi: 10.1016/b978-1-4832-2871-6.50022-9. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann N., Rasmussen H. Calcium, manganese and hepatic gluconeogenesis. Biochim Biophys Acta. 1970 Oct 27;222(1):41–52. doi: 10.1016/0304-4165(70)90349-1. [DOI] [PubMed] [Google Scholar]
- Haugaard N., Haugaard E. S., Lee N. H. Role of magnesium, phosphate and ATP in the regulation of calcium uptake by rat-liver mitochondria. Proc K Ned Akad Wet C. 1969;72(1):1–15. [PubMed] [Google Scholar]
- Nadarajah A., Leese B., Joplin G. F. Triton X-100 scintillant for counting calcium-45 in biological fluids. Int J Appl Radiat Isot. 1969 Oct;20(10):733–735. doi: 10.1016/0020-708x(69)90071-4. [DOI] [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Effect of acetylcholine on the efflux of calcium-45 from the perfused cat submandibular gland. Acta Physiol Scand. 1971 Jul;82(3):22A–23A. doi: 10.1111/j.1748-1716.1971.tb05028.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Excretion of magnesium, calcium, and inorganic phosphate by the cat submandibular gland. Pflugers Arch. 1970;318(1):63–77. doi: 10.1007/BF00588543. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Initiation of salt and water transport in mammalian salivary glands by acetylcholine. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):307–314. doi: 10.1098/rstb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H., Thorn N. A. Secretory potentials, secretory rate and water permeability of the duct system in the cat submandibular gland during perfusion with calcium-free Locke's solution. Acta Physiol Scand. 1967 Oct-Nov;71(2):203–210. doi: 10.1111/j.1748-1716.1967.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger Z., Naim E., Lasser M. ATP-dependent calcium uptake by microsomal preparations from rat parotid and submaxillary glands. Biochim Biophys Acta. 1970 Apr 21;203(2):326–334. doi: 10.1016/0005-2736(70)90147-1. [DOI] [PubMed] [Google Scholar]
- Weber A. Regulatory mechanisms ofthe calcium transport system of ramented rabbit sarcoplasmic rticulum. II. Inhibition of outflux in calcium-free media. J Gen Physiol. 1971 Jan;57(1):64–70. doi: 10.1085/jgp.57.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]


