Abstract
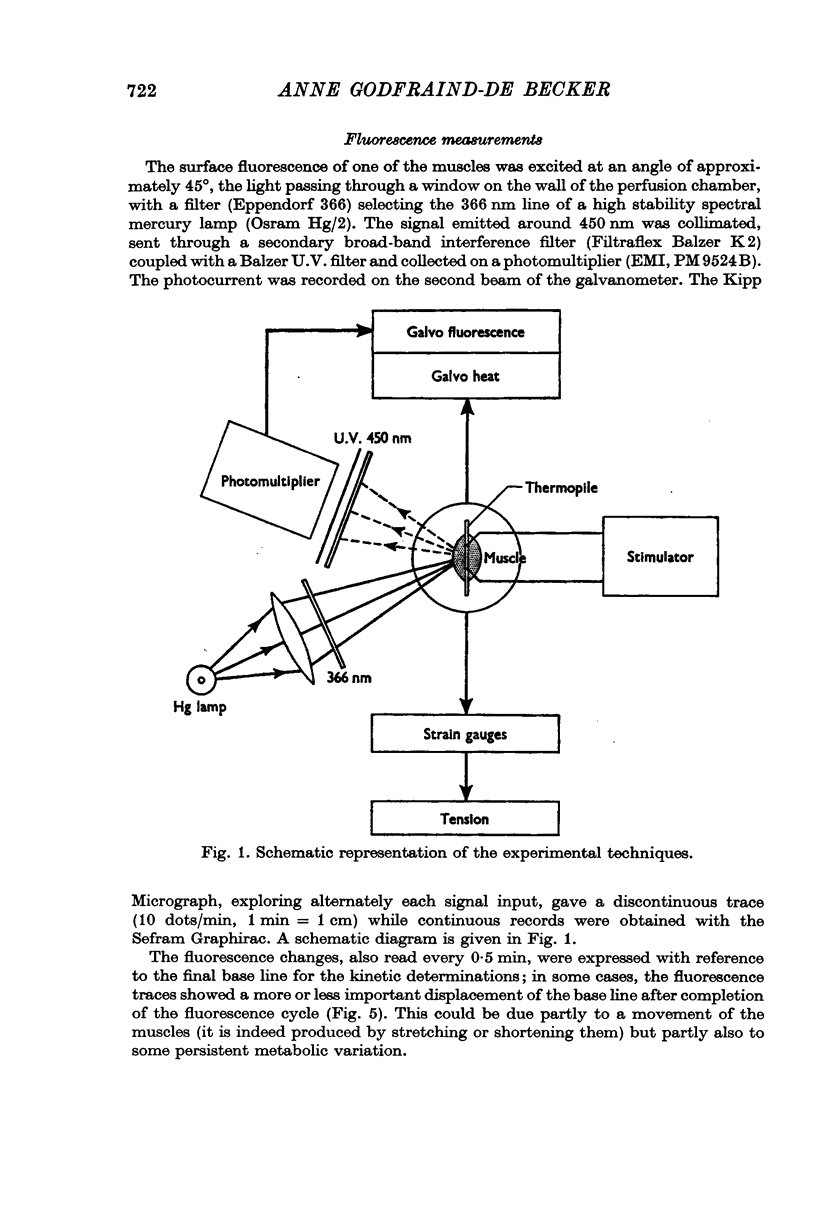
1. The time course of the aerobic recovery following a 0·5 sec tetanus at 20° C of the sartorius muscle of the toad Bufo bufo, equilibrated in bicarbonate-CO2 Ringer solution, has been followed by recording simultaneously the heat production and the fluorescence excited by ultra-violet light at 366 nm.
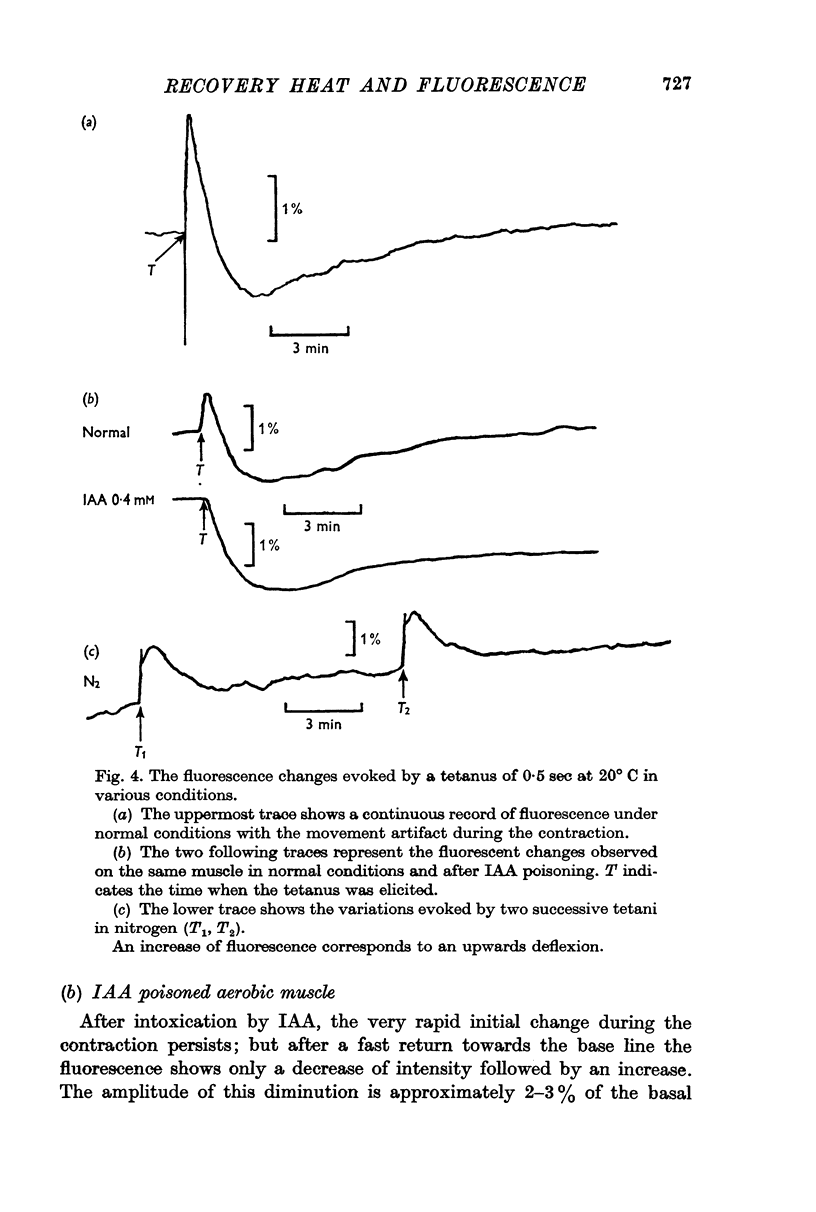
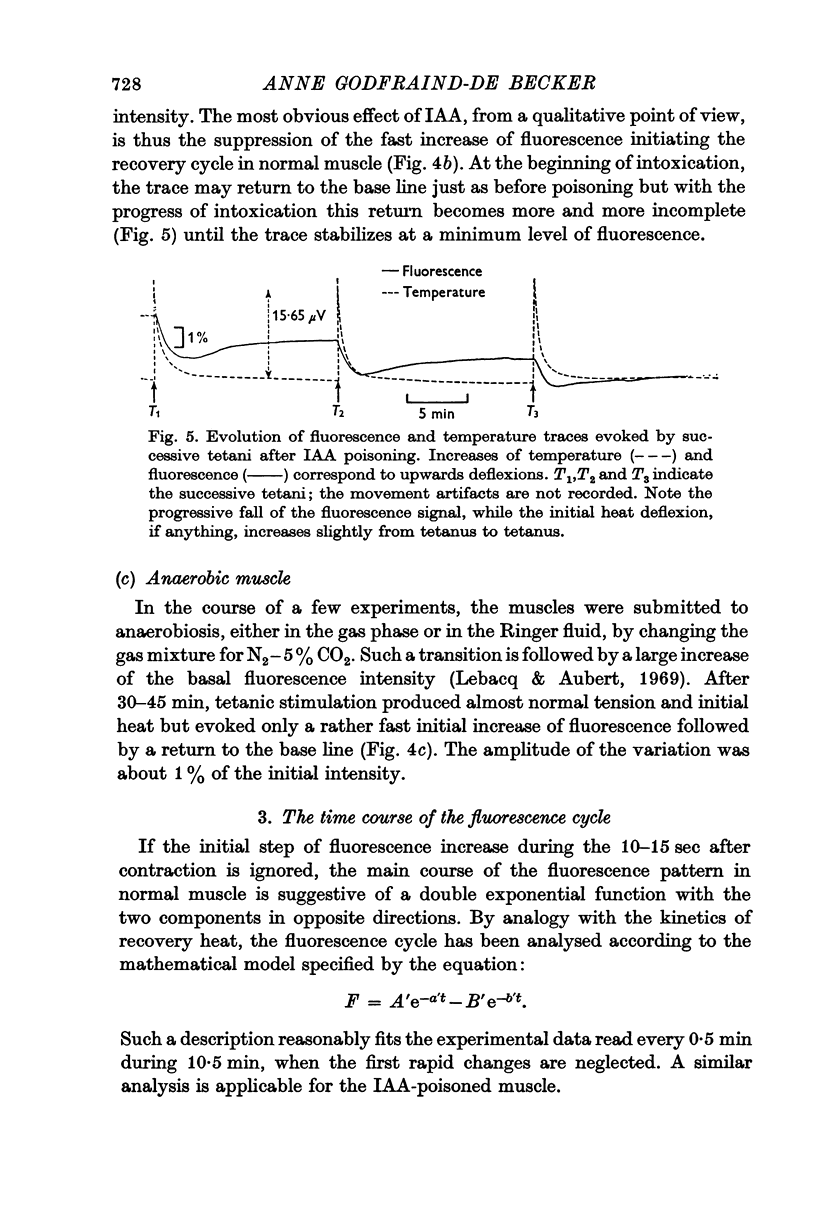
2. The fluorescence light emitted in these conditions in the region of 450 nm monitors the state of oxidation-reduction of the nicotinamideadenine dinucleotides (NAD+-NADH). After a short tetanus, the cycle evoked consists of an initial increase of the fluorescence (reduction of NAD+) followed by a long lasting phase of decreased light emission. This includes an early period of oxidation of NADH succeeded by a slow reduction of the NAD+ formed in excess over the resting state. After iodoacetate, the initial reduction is suppressed.
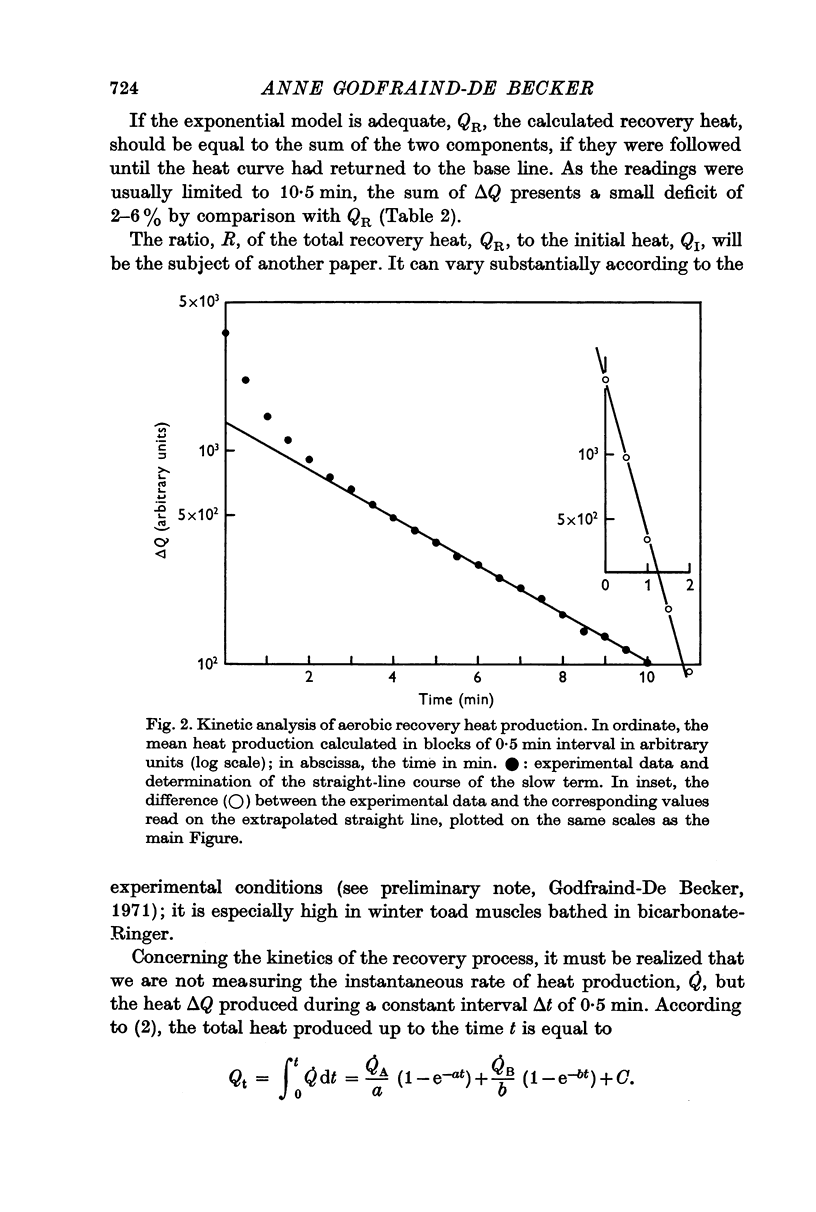
3. The time course of both fluorescence and heat production may be analysed into a rapid and a slow component by a double exponential model.
4. The time courses of the aerobic recovery heat and of the fluorescence changes are similar after five minutes, but differ in their fast components. IAA significantly increases the rate constants of the fast terms of both monitors.
5. The slow component is mainly related to aerobic processes while the fast one is due to both oxidative and glycolytic reactions occurring simultaneously.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauser B., Bücher Th, Dolivo M. Redox transitions of cytochromes and pyridine nucleotides upon stimulation of an isolated rat ganglion. FEBS Lett. 1970 Jun 27;8(5):297–300. doi: 10.1016/0014-5793(70)80291-5. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Chance B., Cohen P., Jobsis F., Schoener B. Localized Fluorometry of Oxidation-Reduction States of Intracellular Pyridine Nucleotide in Brain and Kidney Cortex of the Anesthetized Rat. Science. 1962 Apr 27;136(3513):325–325. doi: 10.1126/science.136.3513.325. [DOI] [PubMed] [Google Scholar]
- Godfraind-de Becker A., Aubert X. Cinétique du métabolisme de recouvrement du muscle strié de batrachiens analysé par la thermogénèse et la fluorescence. J Physiol (Paris) 1967;59(4 Suppl):413–415. [PubMed] [Google Scholar]
- Godfraind-de Becker A. The ratio of oxidative recovery to initial heat in toad sartorius. J Physiol. 1971;217 (Suppl):63P–64P. [PubMed] [Google Scholar]
- Godfraind-de Becker A. Thermogénèse et variations de fluorescence post-contractiles du muscle strié de batracien. J Physiol (Paris) 1968;60 (Suppl 2):362–363. [PubMed] [Google Scholar]
- HILL A. V. The negative delayed heat production in stimulated muscle. J Physiol. 1961 Sep;158:178–196. doi: 10.1113/jphysiol.1961.sp006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree W., Hill A. V. The recovery heat-production in muscle. J Physiol. 1922 Jul 21;56(5):367–381. doi: 10.1113/jphysiol.1922.sp002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The energy degraded in the recovery processes of stimulated muscles. J Physiol. 1913 Mar 3;46(1):28–80. doi: 10.1113/jphysiol.1913.sp001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. The time course of the oxygen consumption of stimulated frog's muscle. J Physiol. 1940 May 14;98(2):207–227. doi: 10.1113/jphysiol.1940.sp003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOBSIS F. F. Spectrophotometric studies on intact muscle. II. Recovery from contractile activity. J Gen Physiol. 1963 May;46:929–969. doi: 10.1085/jgp.46.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen E., Kohen C., Thorell B., Akerman L. Kinetics of the fluorescence response to microelectrophoretically introduced metabolites in the single living cell. Biochim Biophys Acta. 1968 Apr 16;158(1):185–188. doi: 10.1016/0304-4165(68)90095-0. [DOI] [PubMed] [Google Scholar]
- Lebacq J., Aubert X. Etude cinétique de la fluorescence native du Sartorius de grenouille isolé, pendant et après anaéroboise. Arch Int Physiol Biochim. 1969 May;77(2):337–337. [PubMed] [Google Scholar]
- Williamson J. R., Jamieson D. Dissociation of the inotropic from the glycogenolytic effect of epinephrine in the isolated rat heart. Nature. 1965 Apr 24;206(982):364–367. doi: 10.1038/206364a0. [DOI] [PubMed] [Google Scholar]


