Abstract
1. The efflux of calcium, as the isotope 45Ca, has been investigated from single muscle fibres from the barnacle Balanus nubilus and from the crab Maia squinado.
2. If the isotope was initially injected with sufficient calcium (5-65 mM) to cause a contraction, the efflux did not follow first order kinetics. There was an early rapid phase which reached a peak after 5-10 min and then declined slowly over a period of 50-150 min to a low residual value.
3. Injection of the isotope with the calcium-binding agent EGTA, so that the injected free calcium concentration was ca. 2 × 10-8 M, abolished the initial rapid loss of calcium. The efflux rose to give a steady value after 10-15 min and its magnitude was similar to the value of the residual efflux.
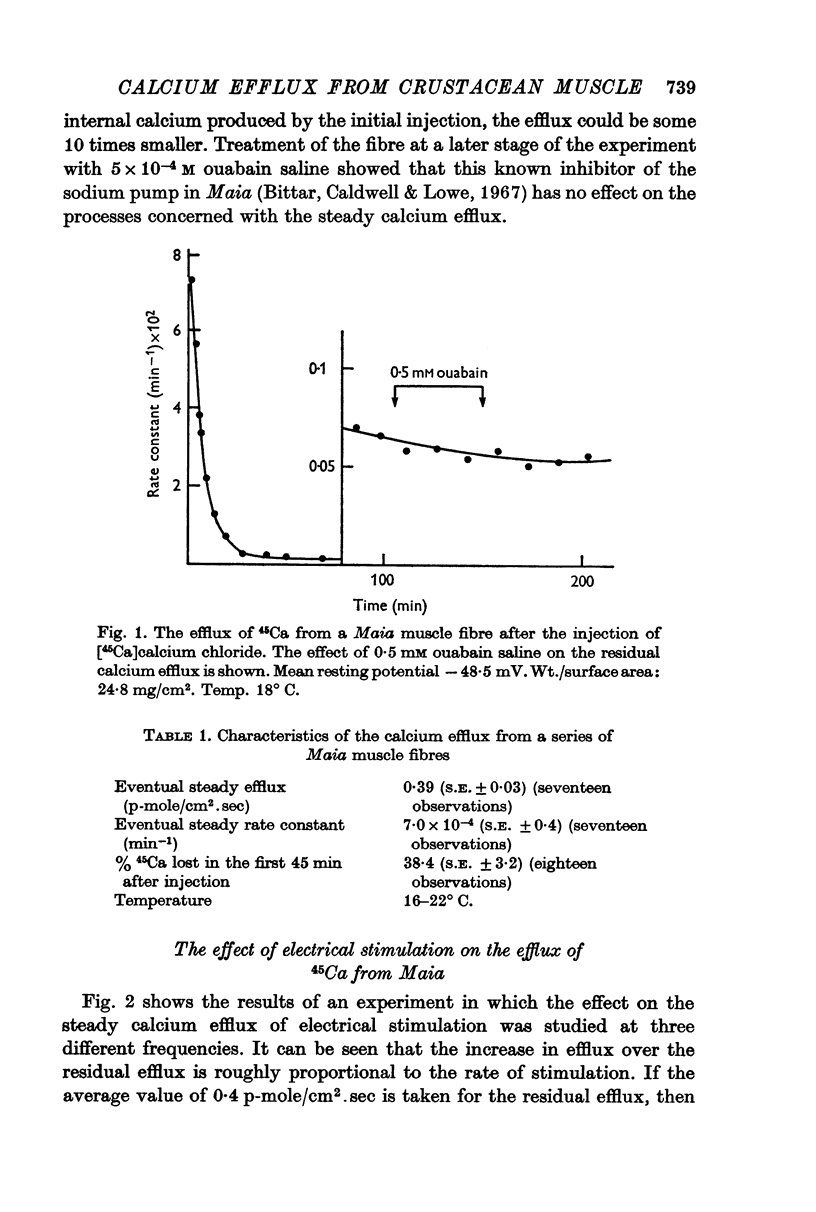
4. The rate constant for the low residual loss was ca. 7 × 10-4 min-1 for Maia and ca. 17 × 10-4 min-1 for Balanus. The rate constant predicted a calcium efflux of 0·4 p-mole/cm2.sec for Maia and 1-2 p-mole/cm2.sec for Balanus at 16-25° C based on the total fibre calcium concentration.
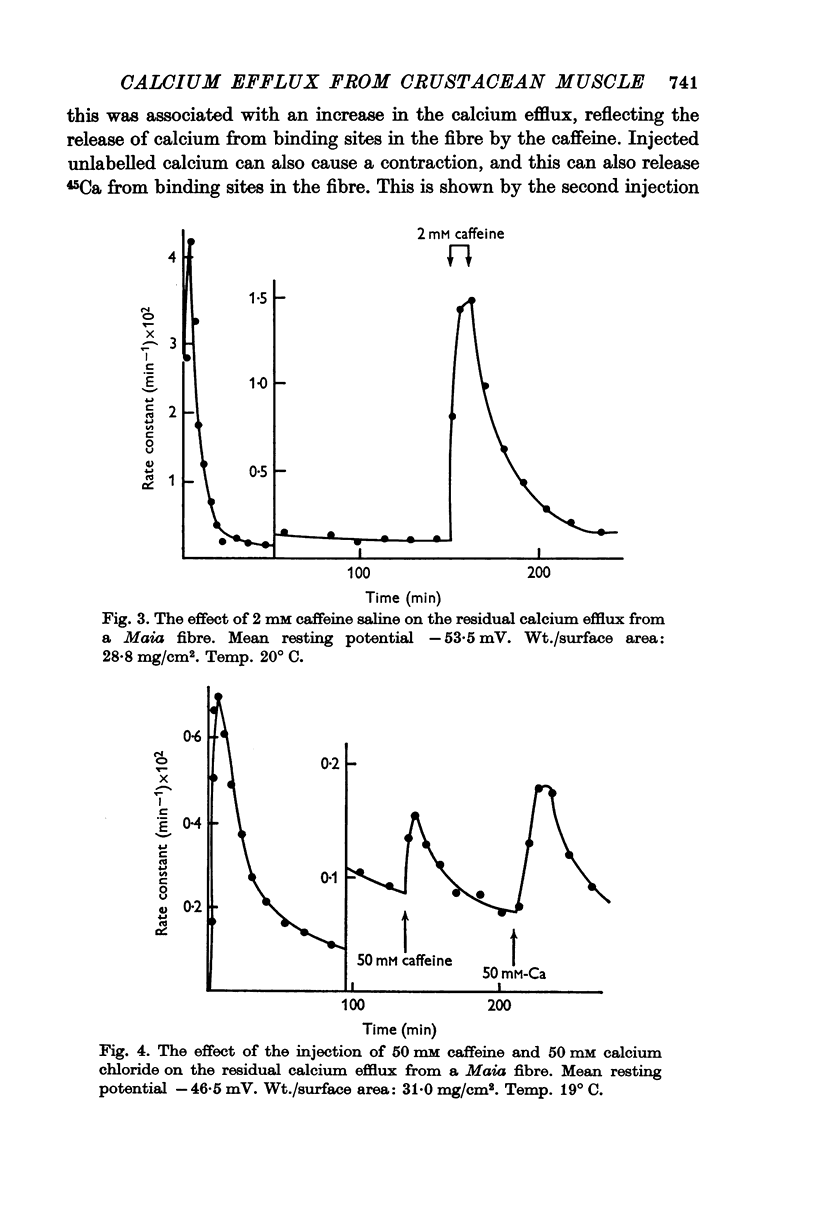
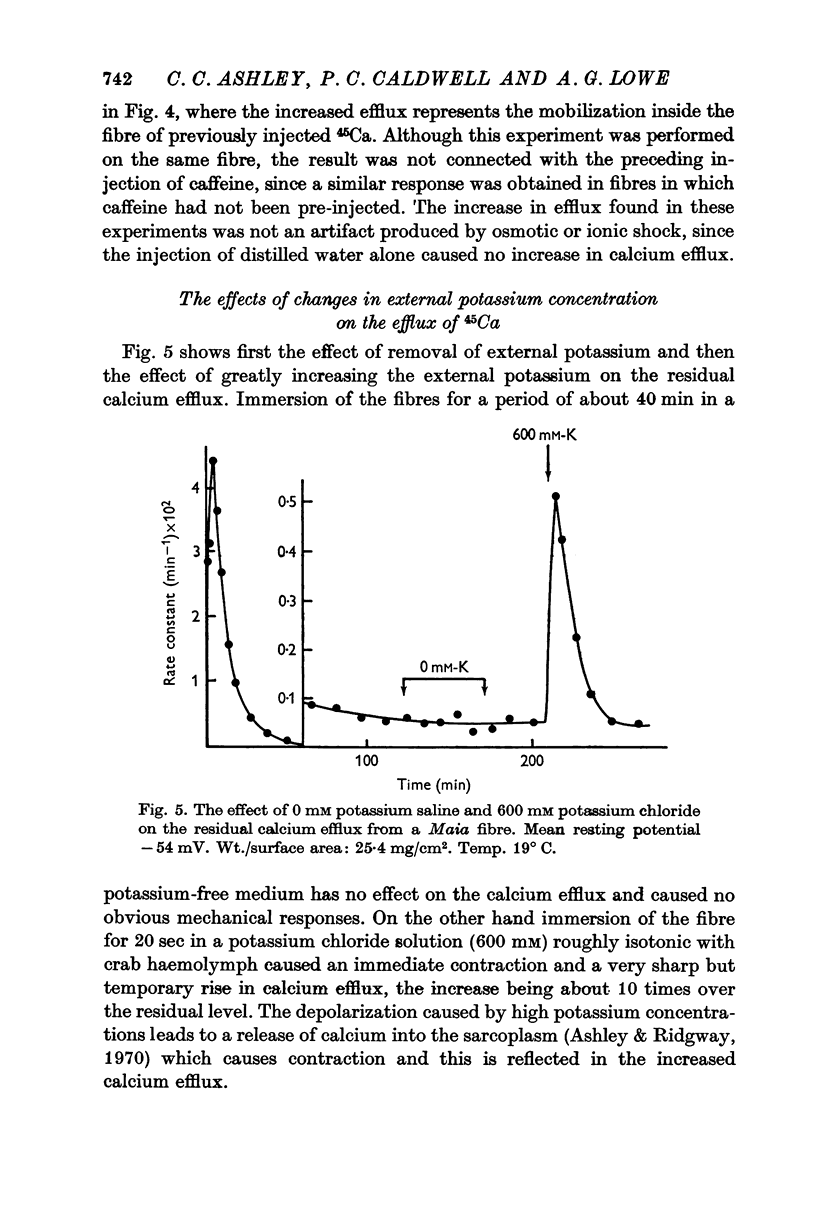
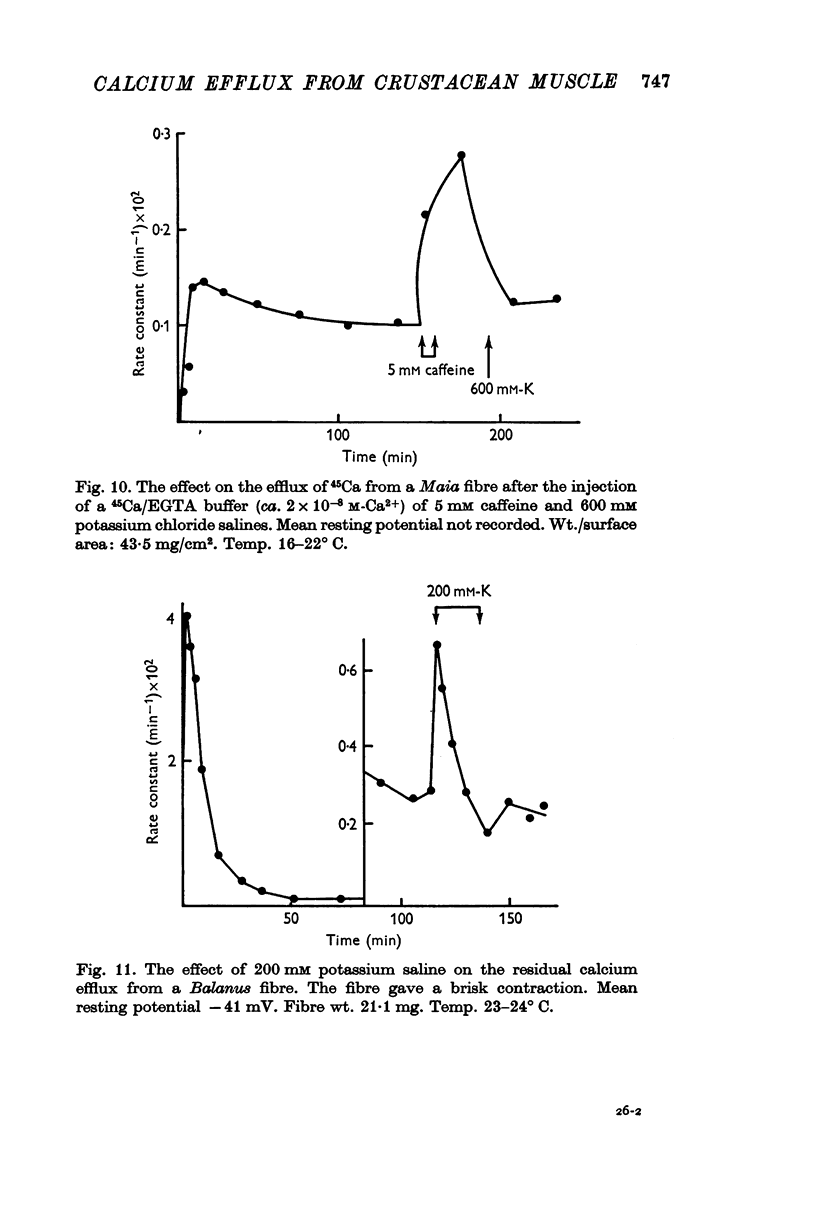
5. The residual calcium efflux was not affected by 0·5 mM ouabain or 0 potassium salines applied externally. It was stimulated, some 10-15 times in Maia and to a lesser extent in Balanus, by salines containing 600 mM potassium or 2-5 mM caffeine. The increased efflux was associated with a brisk contraction.
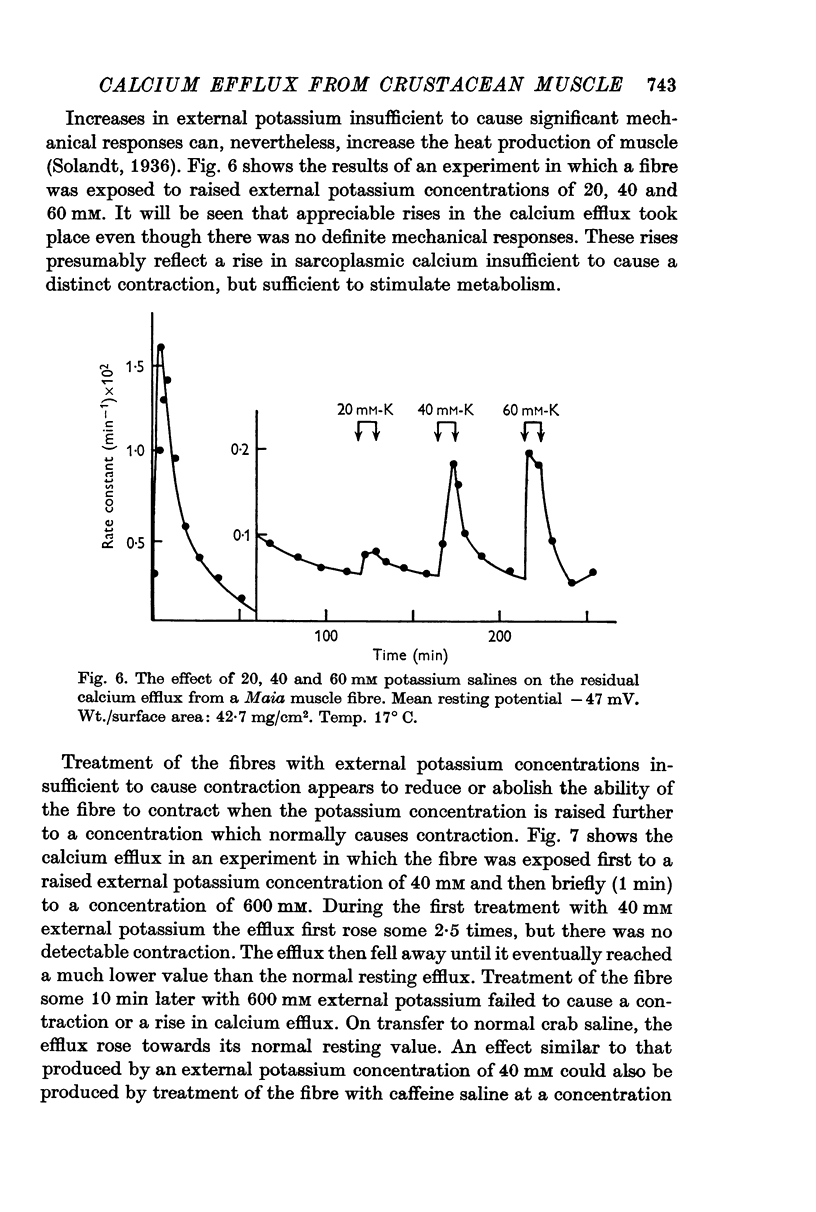
6. External application of salines containing 20, 40 or 60 mM potassium or 0·5 mM caffeine in Maia produced some stimulation of the residual efflux but no visible contraction.
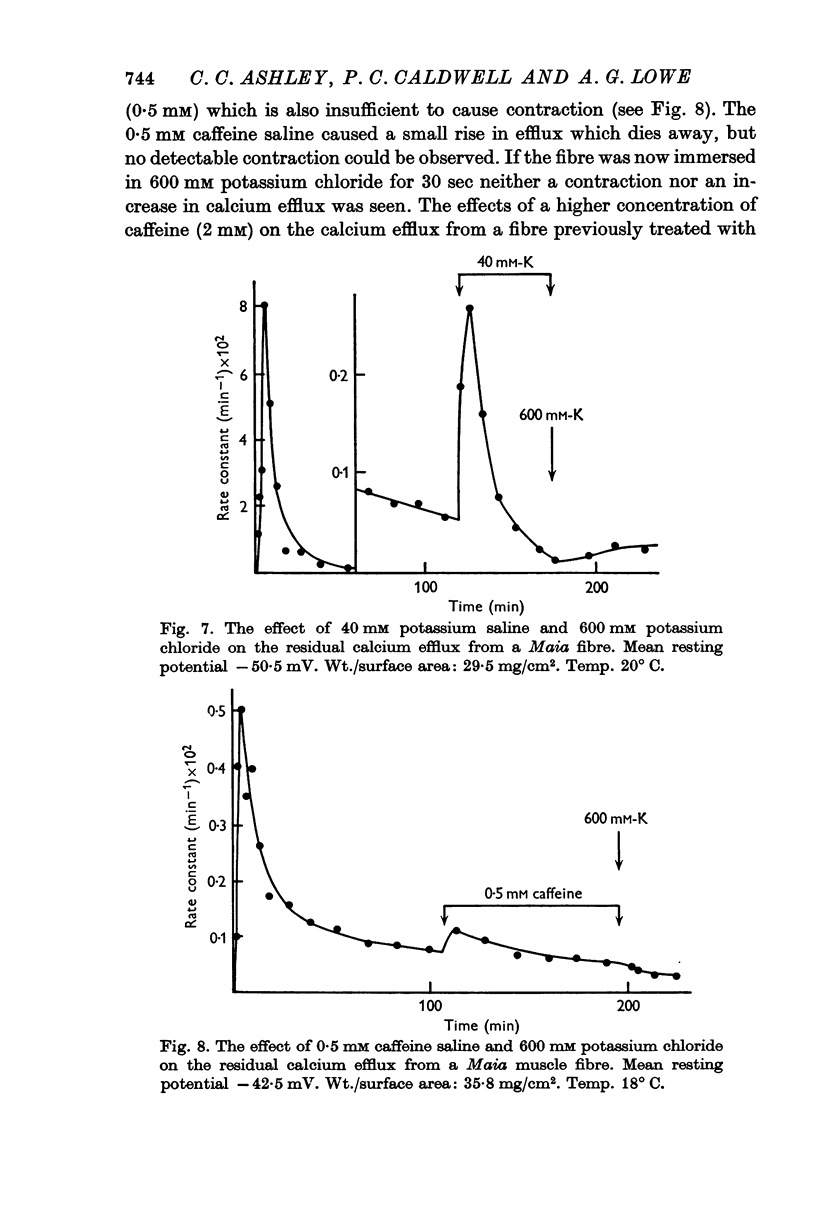
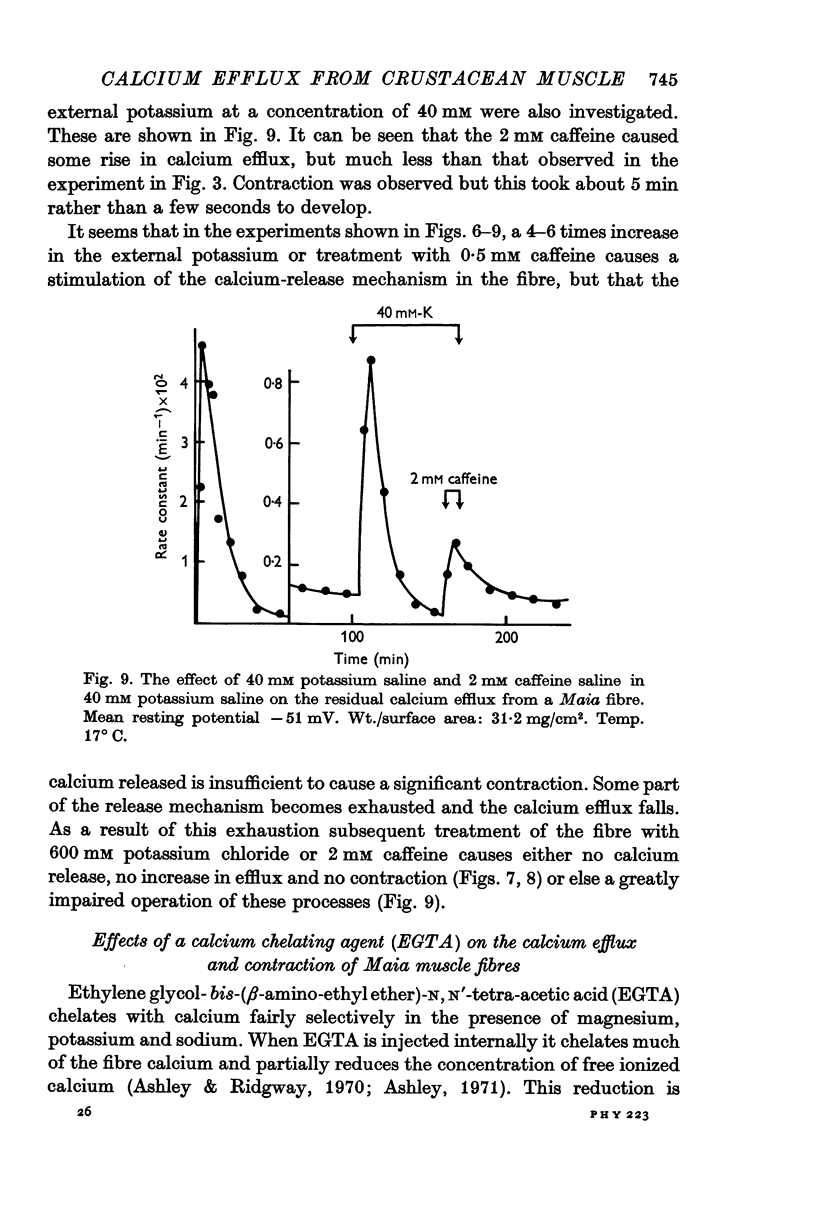
7. Pre-treatment of Maia fibres with 40 mM potassium or 0·5 mM caffeine salines abolished the ability of the fibres to respond to higher concentrations of these agents. A depletion of a releasable calcium fraction by these subthreshold stimuli could explain this phenomenon.
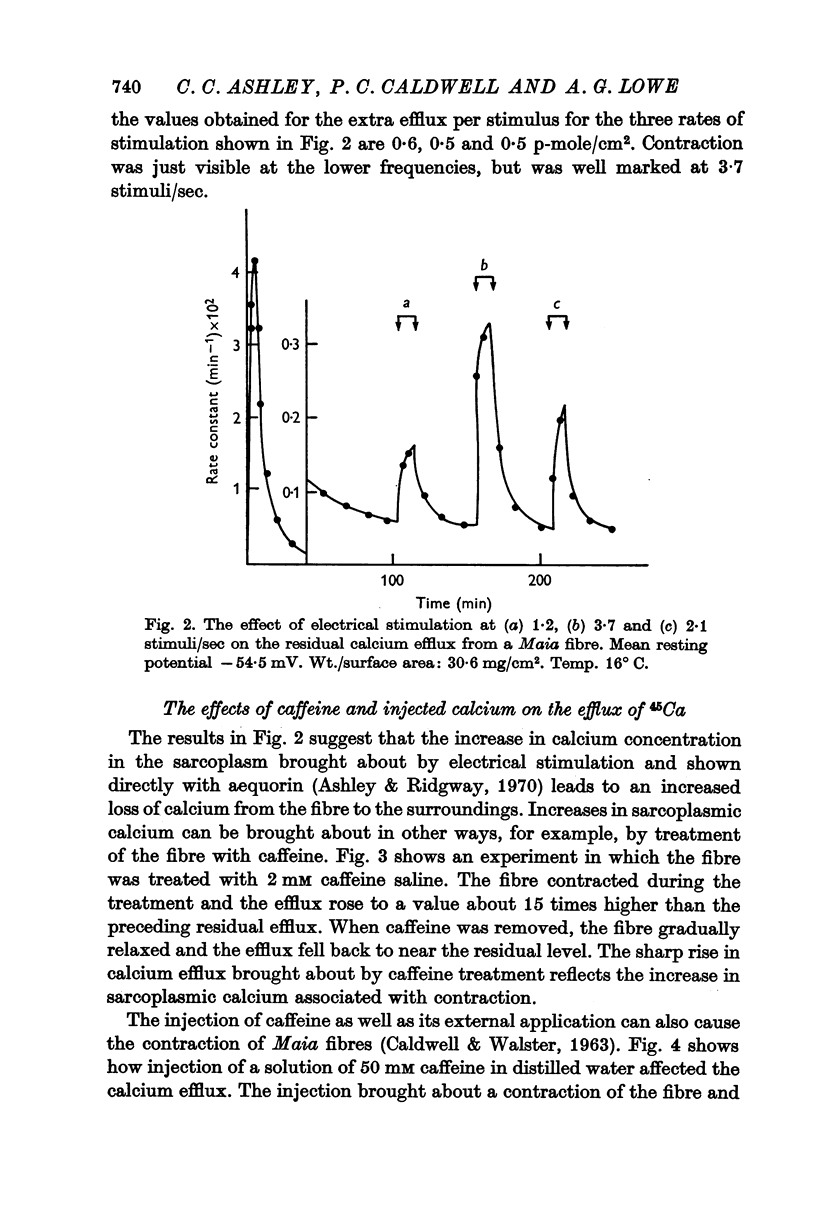
8. Electrical stimulation, the injection of 50 mM calcium chloride or 50 mM caffeine produced an elevated calcium efflux which was associated with a contraction.
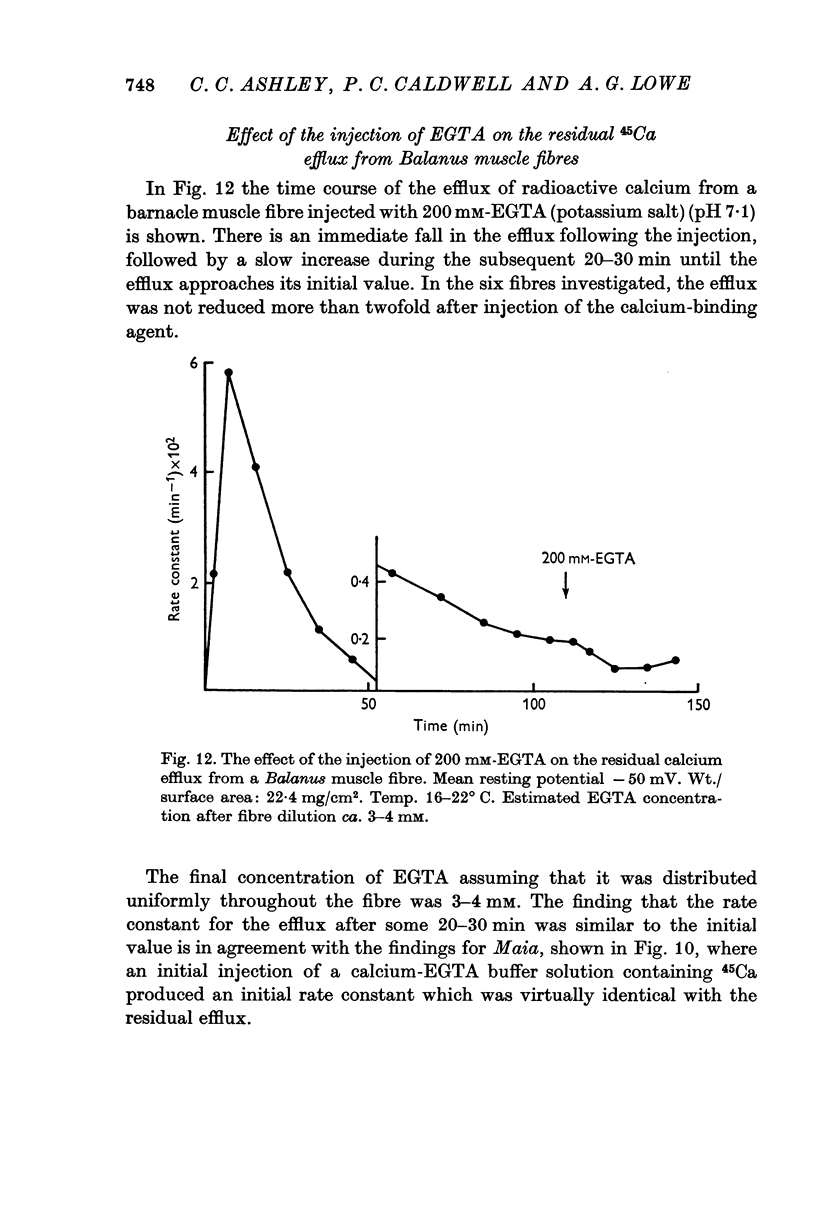
9. Intracellular injections of EGTA only lowered the residual efflux by up to half its initial value. This suggests that calcium can be released rapidly within these muscle fibres and that the sarcoplasmic calcium concentration is not much altered from its normal value by the injection.
10. The experiments suggest that in Maia, changes in the calcium efflux reflect in magnitude, but not in time course, the internal calcium changes which can be observed with the calcium-sensitive protein, aequorin.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. An estimate of calcium concentration changes during the contraction of single muscle fibres. J Physiol. 1970 Sep;210(2):133P–134P. [PubMed] [Google Scholar]
- Ashley C. C. Calcium and the activation of skeletal muscle. Endeavour. 1971 Jan;30(109):18–25. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. Simultaneous recording of membrane potential, calcium transient and tension in single muscle fibers. Nature. 1968 Sep 14;219(5159):1168–1169. doi: 10.1038/2191168a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C. The role of cell calcium in the contraction of single cannulated muscle fibers. Am Zool. 1967 Aug;7(3):647–659. doi: 10.1093/icb/7.3.647. [DOI] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Sodium and potassium fluxes in isolated barnacle muscle fibers. J Gen Physiol. 1968 Apr;51(4):445–477. doi: 10.1085/jgp.51.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. CALCIUM AND THE CONTRACTION OF MAIA MUSCLE FIBRES. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:512–516. doi: 10.1098/rspb.1964.0066. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C. CALCIUM AND THE CONTRACTION OF MAIA MUSCLE FIBRES. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:512–516. doi: 10.1098/rspb.1964.0066. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Liquid junction potentials and their effect on potential measurements in biological systems. Int Rev Cytol. 1968;24:345–371. doi: 10.1016/s0074-7696(08)61402-3. [DOI] [PubMed] [Google Scholar]
- Connolly R., Gough W., Winegrad S. Characteristics of the isometric twitch of skeletal muscle immediately after a tetanus. A study of the influence of the distribution of calcium within the sarcoplasmic reticulum on the twitch. J Gen Physiol. 1971 Jun;57(6):697–709. [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. UBER DEN MECHANISMUS DES CALCIUMTRANSPORTES DURCH DIE MEMBRANEN DES SARKOPLASMATISCHEN RETICULUMS. Biochem Z. 1963 Oct 14;339:94–111. [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. [The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting]. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Lloyd S., Pickford M. The effect of oxytocin and adrenaline on blood flow in the hind limb of the dog following chronic lumbar sympathectomy. J Physiol. 1967 Sep;192(1):43–52. doi: 10.1113/jphysiol.1967.sp008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. Radiocalcium release by stimulated and potassium-treated sartorius muscles of the frog. J Gen Physiol. 1960 Jan;43:481–493. doi: 10.1085/jgp.43.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solandt D. Y. The effect of potassium on the excitability and resting metabolism of frog's muscle. J Physiol. 1936 Feb 8;86(2):162–170. doi: 10.1113/jphysiol.1936.sp003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. THE REGULATION OF MYOFIBRILLAR ACTIVITY BY CALCIUM. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:489–501. doi: 10.1098/rspb.1964.0063. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968 Jan;51(1):65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]


