Abstract
1. Sarcoplasmic reticulum fragments (SRF) and actomyosin were isolated from skeletal muscle of cats treated either with thyroxine or with placebo tablets, for 5-16 months.
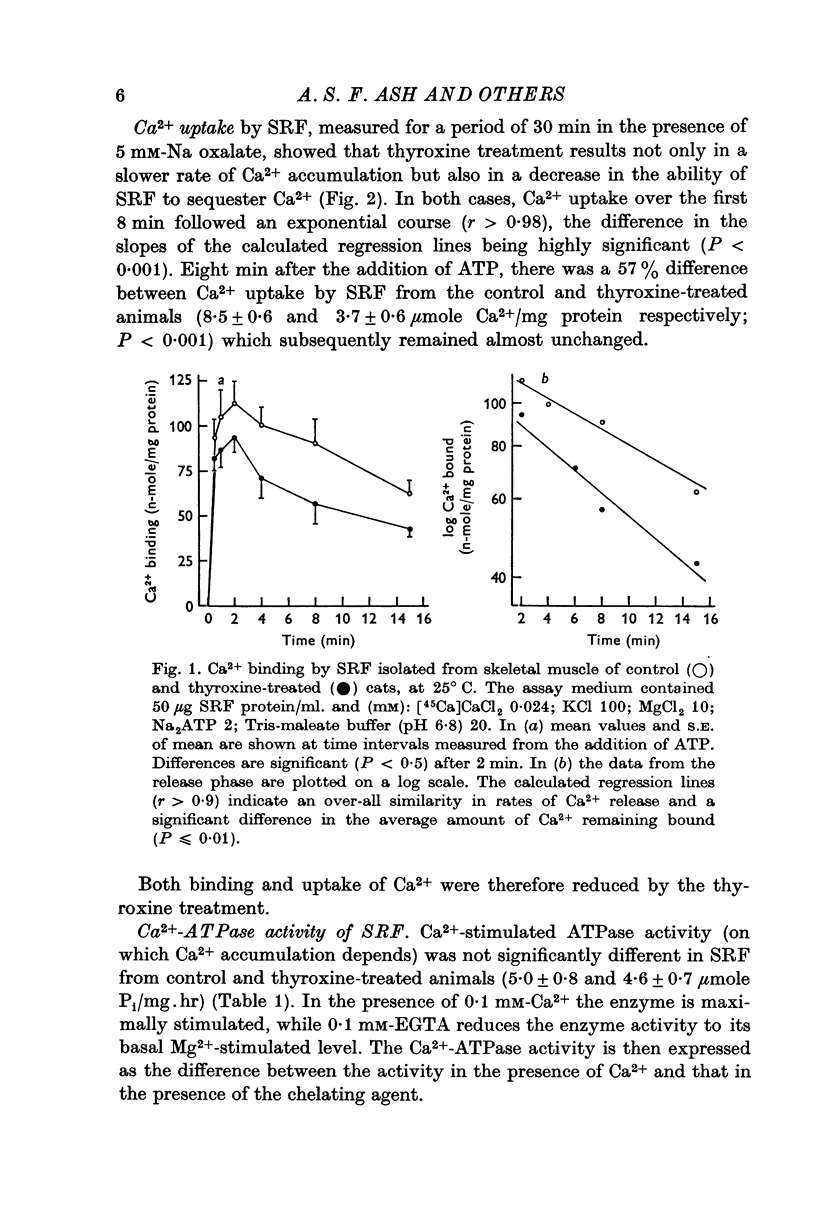
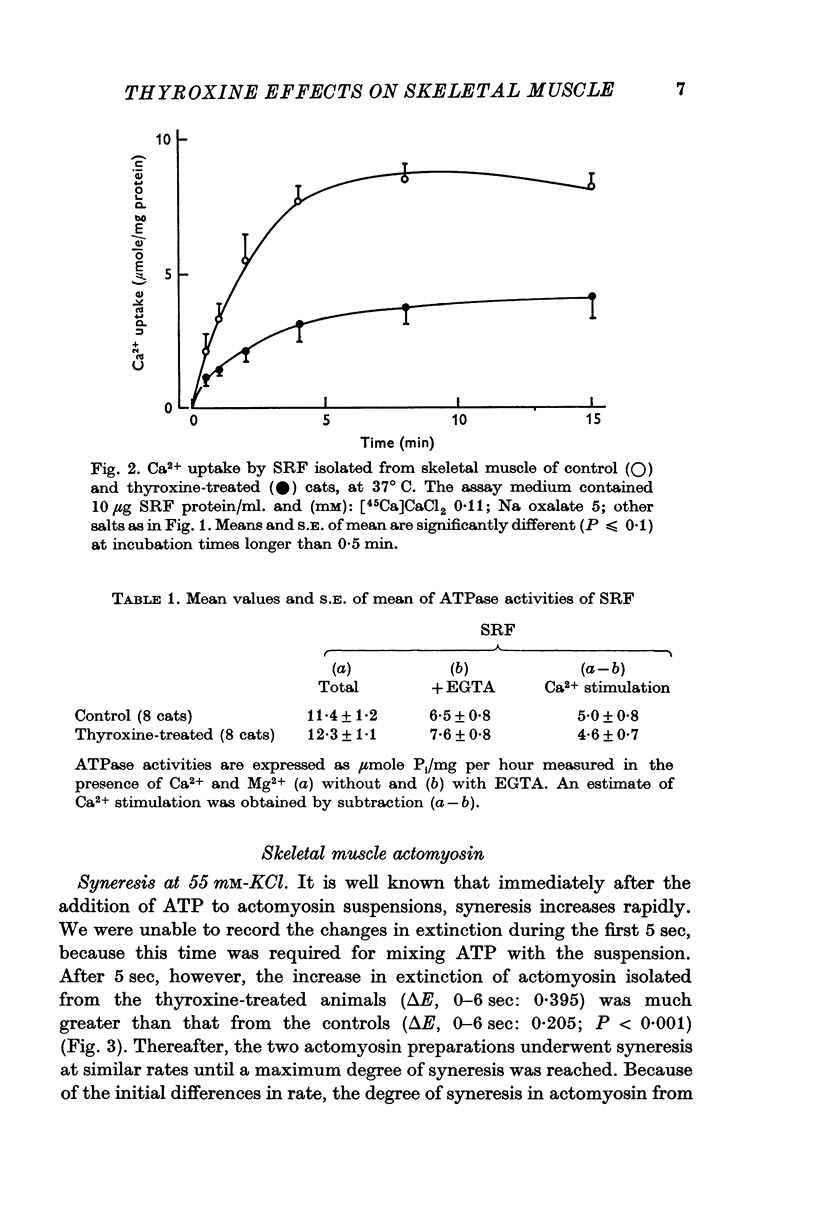
2. Ca2+ uptake and binding by SRF, measured in the presence and absence of oxalate respectively, were reduced by thyroxine treatment.
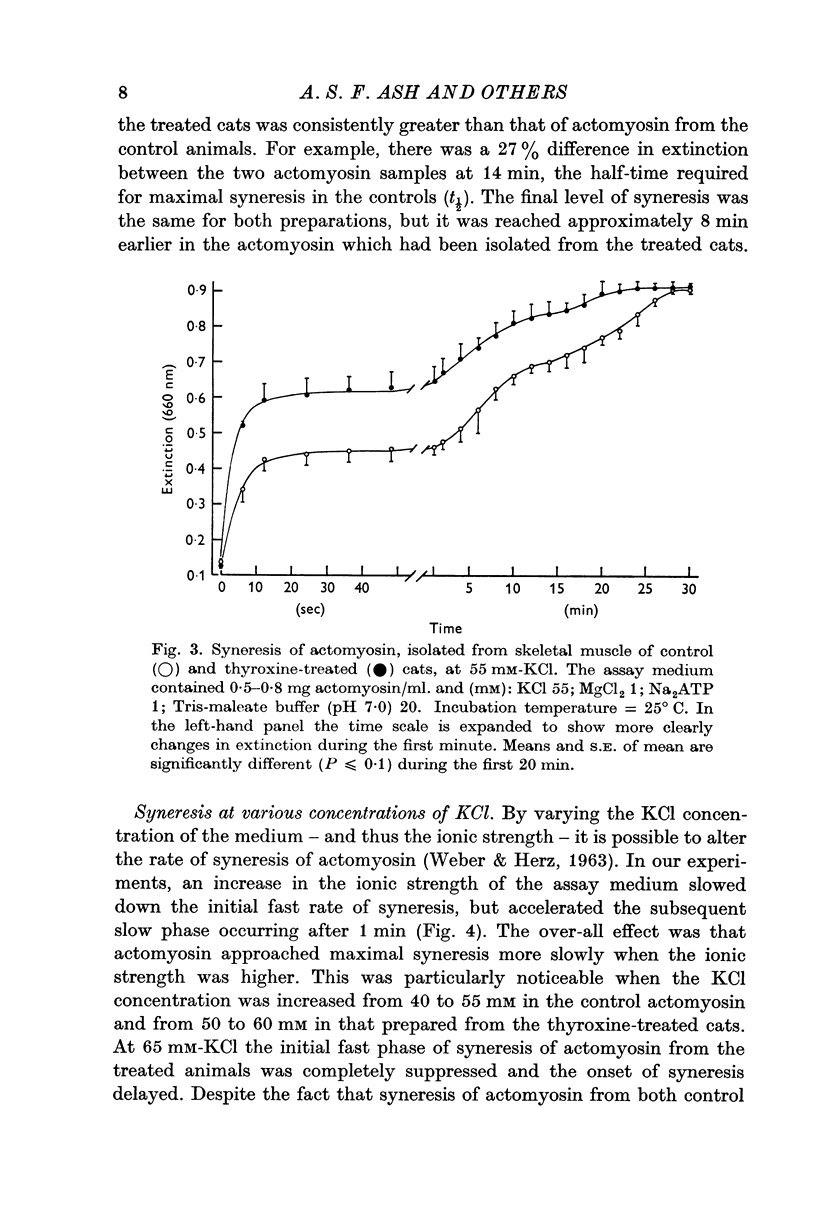
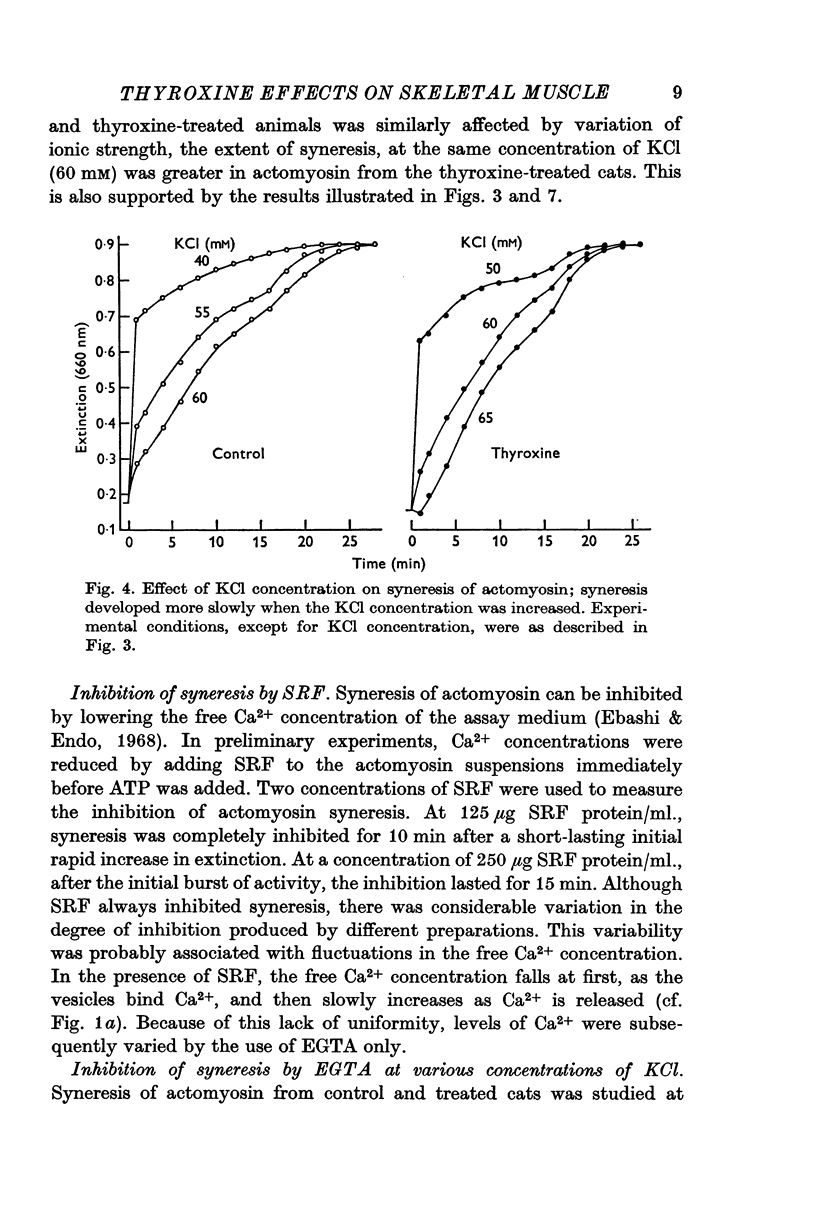
3. Actomyosin from the thyroxine-treated animals underwent ATP-induced syneresis at a faster rate than did that from the controls.
4. Syneresis of the actomyosin preparations from controls and treated animals was inhibited to the same extent by EGTA when rates of syneresis were made the same by adjustment of the KCl concentration in the assay media. In contrast, at any given KCl concentration, syneresis of actomyosin from thyroxine-treated animals was inhibited to a lesser extent by EGTA.
5. At the end of the isolation procedure, the amount of Ca2+ remaining in the actomyosin suspension was similar for both treated and control animals.
6. It was concluded that the effect of thyroxine on skeletal muscle may be the result of an action on both the sarcoplasmic reticulum and the contractile protein complex.
Full text
PDF




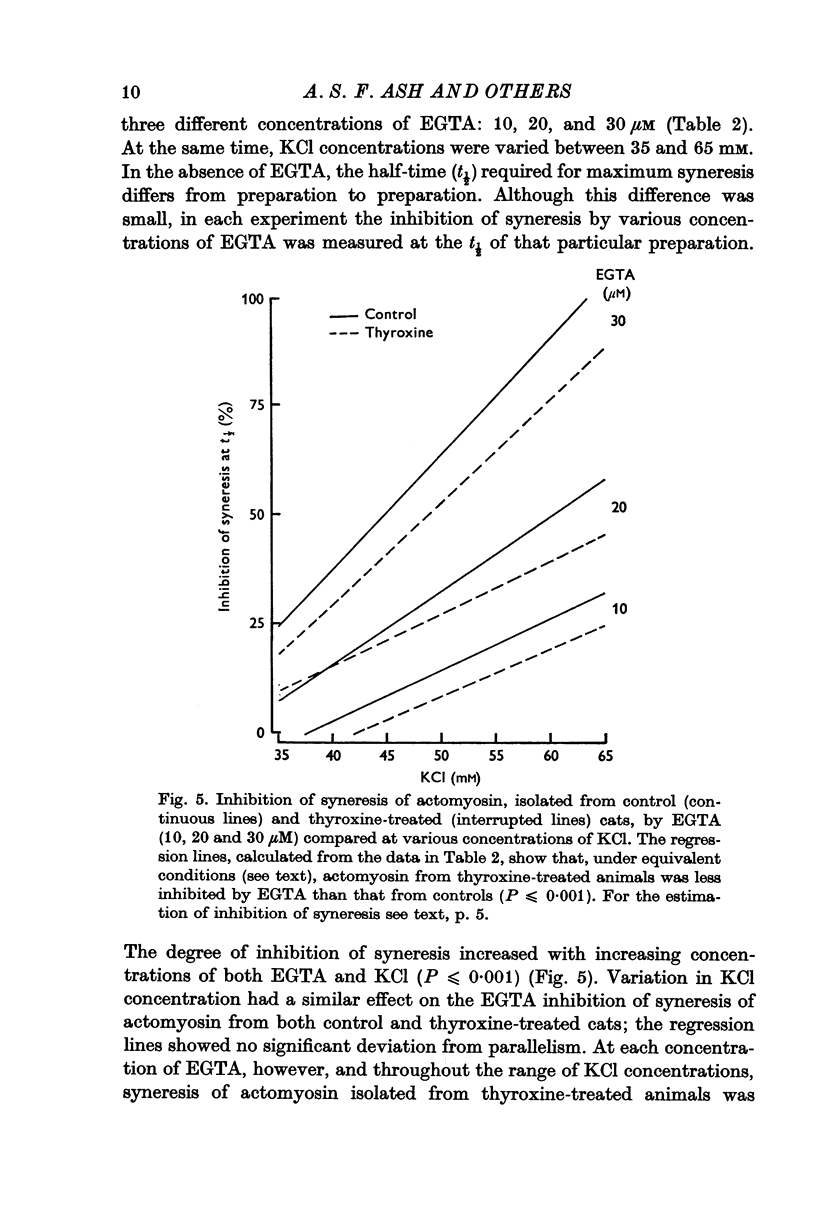
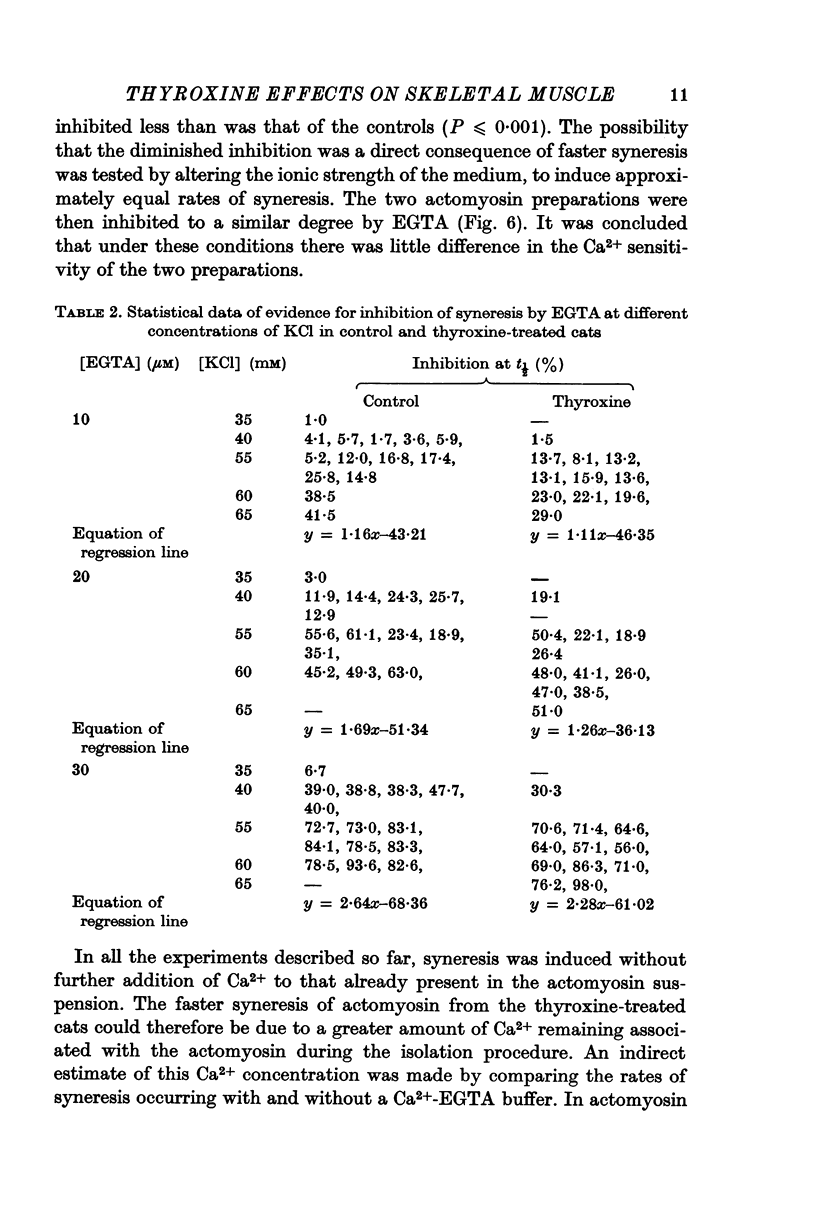
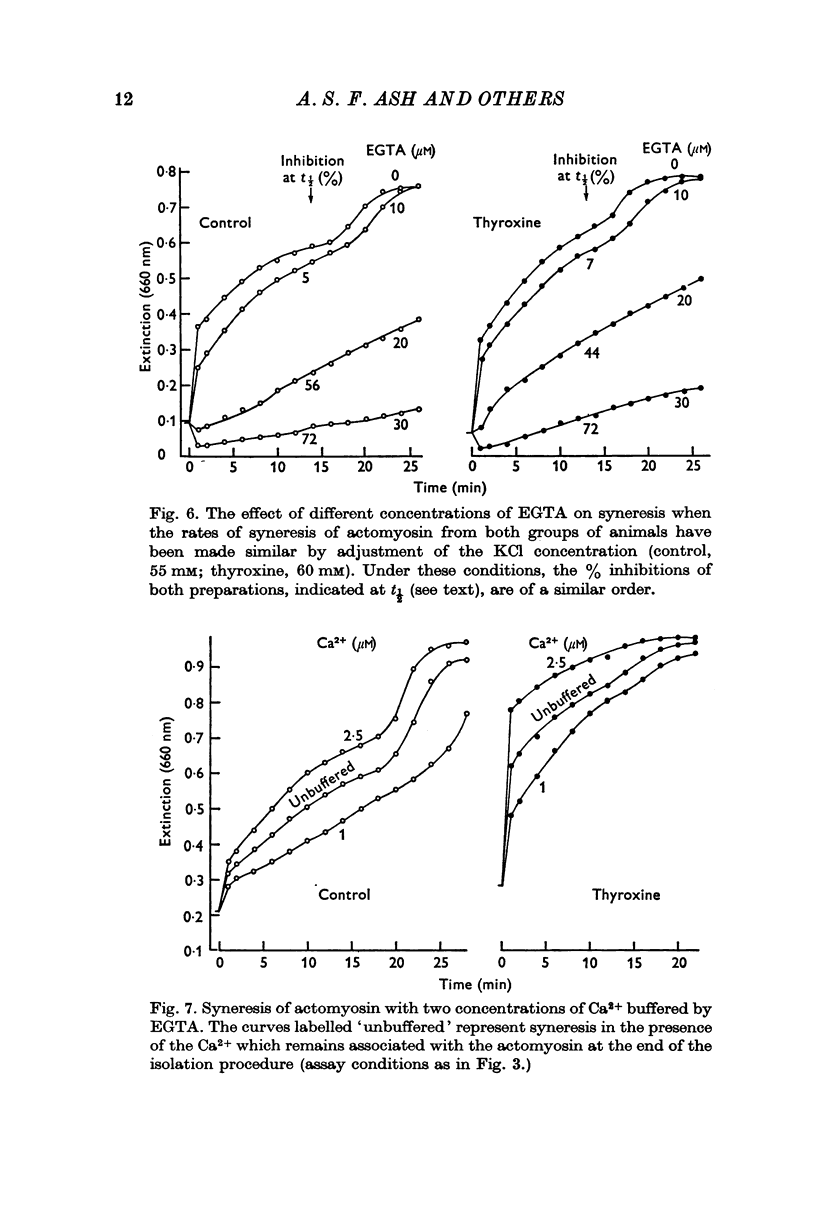







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Watanabe S. A study of troponin, a myofibrillar protein from rabbit skeletal muscle. J Biol Chem. 1968 Nov 10;243(21):5670–5678. [PubMed] [Google Scholar]
- BAIRD G. D., PERRY S. V. The inhibitory action of relaxing-factor preparation on the myofibrillar adenosine triphosphatase. Biochem J. 1960 Nov;77:262–271. doi: 10.1042/bj0770262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Carvalho A. P. Effects of potentiators of muscular contraction on binding of cations by sarcoplasmic reticulum. J Gen Physiol. 1968 Mar;51(3):427–442. doi: 10.1085/jgp.51.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLEDINE W. S., PITTRIVERS R., STANBURY J. B. Nature and transport of the iodinated substances of the blood of normal subjects and of patients with thyroid disease. J Clin Endocrinol Metab. 1955 Jun;15(6):724–731. doi: 10.1210/jcem-15-6-724. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Baskin R. J. Ultrastructure of sarcoplasmic reticulum preparations. J Cell Biol. 1969 Jul;42(1):296–307. doi: 10.1083/jcb.42.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI F., YAMANOUCHI I. CALCIUM ACCUMULATION AND ADENOSINETRIPHOSPHATASE OF THE RELAXING FACTOR. J Biochem. 1964 May;55:504–509. [PubMed] [Google Scholar]
- EBASHI S. Calcium binding activity of vesicular relaxing factor. J Chir (Paris) 1961 Sep;82:236–244. doi: 10.1093/oxfordjournals.jbchem.a127439. [DOI] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- ENDO M. THE SUPERPRECIPITATION OF ACTOMYOSIN AND ITS ATPASE ACTIVITY IN LOW CONCENTRATION OF ATP. J Biochem. 1964 Jun;55:614–622. doi: 10.1093/oxfordjournals.jbchem.a127934. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A., Ebashi F. Troponin. I. Preparation and physiological function. J Biochem. 1968 Oct;64(4):465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Lipmann F. ADENOSINE TRIPHOSPHATE-LINKED CONCENTRATION OF CALCIUM IONS IN A PARTICULATE FRACTION OF RABBIT MUSCLE. J Cell Biol. 1962 Sep 1;14(3):389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F., Gertz E. W., Briggs F. N. The effect of quinidine on calcium accumulation by isolated sarcoplasmic reticulum of skeletal and cardiac muscle. J Gen Physiol. 1968 Dec;52(6):955–968. doi: 10.1085/jgp.52.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- GROB D. Myopathies and their relation to thyroid disease. N Y State J Med. 1963 Jan 15;63:218–228. [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Cassens R. G., Hoekstra W. G., Briskey E. J. Purification and ultrastructural properties of the calcium accumulating membranes in isolated sarcoplasmic reticulum preparations from skeletal muscle. J Cell Physiol. 1969 Aug;74(1):37–50. doi: 10.1002/jcp.1040740106. [DOI] [PubMed] [Google Scholar]
- HAVARD C. W., CAMPBELL E. D., ROSS H. B., SPENCE A. W. Electromyographic and histological findings in the muscles of patients with thyrotoxicosis. Q J Med. 1963 Apr;32:145–163. [PubMed] [Google Scholar]
- HOCH F. L. Biochemical actions of thyroid hormones. Physiol Rev. 1962 Oct;42:605–673. doi: 10.1152/physrev.1962.42.4.605. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. Structural and enzymatic properties of the calcium transporting membranes of the sarcoplasmic reticulum. Ann N Y Acad Sci. 1966 Jul 14;137(2):1041–1048. doi: 10.1111/j.1749-6632.1966.tb50216.x. [DOI] [PubMed] [Google Scholar]
- Herz R., Weber A., Reiss I. The role of magnesium in the relaxation of myofibrils. Biochemistry. 1969 Jun;8(6):2266–2271. doi: 10.1021/bi00834a005. [DOI] [PubMed] [Google Scholar]
- LIU C. T., OVERMAN R. R. EFFECT OF TOXIC DOSES OF L-THYROXINE ON TISSUE WATER, ELECTROLYTES AND PLASMA PROTEIN IN RATS. Proc Soc Exp Biol Med. 1964 Oct;117:232–236. doi: 10.3181/00379727-117-29544. [DOI] [PubMed] [Google Scholar]
- MACLAGAN N. F., BOWDEN C. H., WILKINSON J. H. The metabolism of thyroid hormones. 2. Detection of thyroxine and tri-iodothyronine in human plasma. Biochem J. 1957 Sep;67(1):5–11. doi: 10.1042/bj0670005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MUSCATELLO U., ANDERSSON-CEDERGREN E., AZZONE G. F. The mechanism of muscle-fiber relaxation adenosine triphosphatase and relaxing activity of the sarcotubular system. Biochim Biophys Acta. 1962 Sep 10;63:55–74. doi: 10.1016/0006-3002(62)90338-4. [DOI] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. Der Einfluss von Oxalat auf den Calcium-Transport isolierter Vesikel des sarkoplasmatischen Reticulum. Biochem Z. 1965 Dec 31;343(4):360–382. [PubMed] [Google Scholar]
- Martonosi A. Sarcoplasmic reticulum. V. The structure of sarcoplasmic reticulum membranes. Biochim Biophys Acta. 1968 Jun 11;150(4):694–704. doi: 10.1016/0005-2736(68)90059-x. [DOI] [PubMed] [Google Scholar]
- NAGAI T., MAKINOSE M., HASSEL BACH W. [The physiological relaxing factor produced by the muscle granules]. Biochim Biophys Acta. 1960 Sep 23;43:223–238. doi: 10.1016/0006-3002(60)90433-9. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Perry S. V. The structure and interactions of myosin. Prog Biophys Mol Biol. 1967;17:325–381. doi: 10.1016/0079-6107(67)90010-7. [DOI] [PubMed] [Google Scholar]
- Smith E. K., Samuel P. D. Abnormalities in the sodium pump of erythrocytes from patients with hyperthyroidism. Clin Sci. 1970 Jan;38(1):49–61. doi: 10.1042/cs0380049. [DOI] [PubMed] [Google Scholar]
- Stewart J. M., Levy H. M. The role of the calcium-troponin-tropomyosin complex in the activation of contraction. J Biol Chem. 1970 Nov 10;245(21):5764–5772. [PubMed] [Google Scholar]
- TIMIRAS P. S., WOODBURY D. M. Effect of thyroid activity on brain function and brain electrolyte distribution in rats. Endocrinology. 1956 Feb;58(2):181–192. doi: 10.1210/endo-58-2-181. [DOI] [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. On the mechanism of the relaxing effect of fragmented sarcoplasmic reticulum. J Gen Physiol. 1963 Mar;46:679–702. doi: 10.1085/jgp.46.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R. The binding of calcium to actomyosin systems in relation to their biological activity. J Biol Chem. 1963 Feb;238:599–605. [PubMed] [Google Scholar]
- WEBER A., WINICUR S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961 Dec;236:3198–3202. [PubMed] [Google Scholar]
- Zaimis E., Papadaki L., Ash A. S., Larbi E., Kakari S., Matthew M., Paradelis A. Cardiovascular effects of thyroxine. Cardiovasc Res. 1969 Apr;3(2):118–133. doi: 10.1093/cvr/3.2.118. [DOI] [PubMed] [Google Scholar]
- de Meis L., Rubin-Altschul M., Machado R. D. Comparative data of Ca2+ transport in brain and skeletal muscle microsomes. J Biol Chem. 1970 Apr 25;245(8):1883–1889. [PubMed] [Google Scholar]


