Abstract
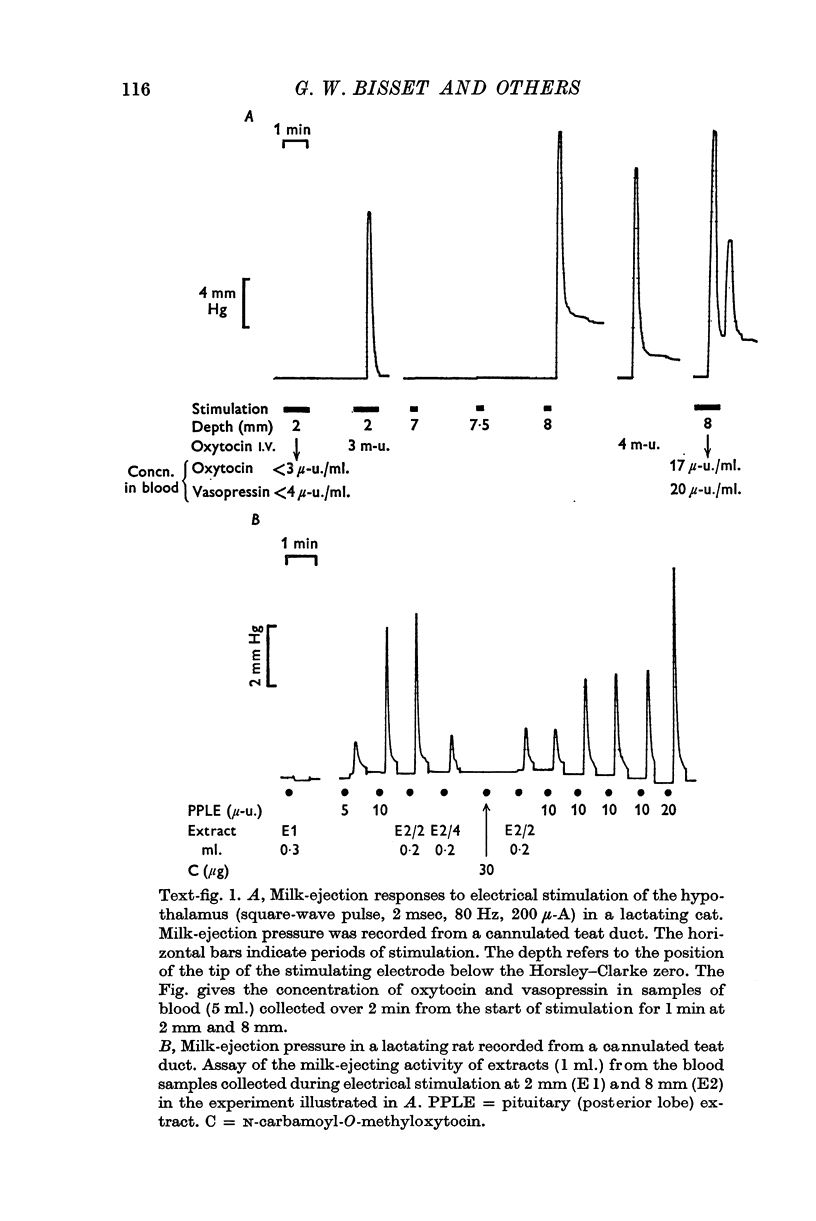
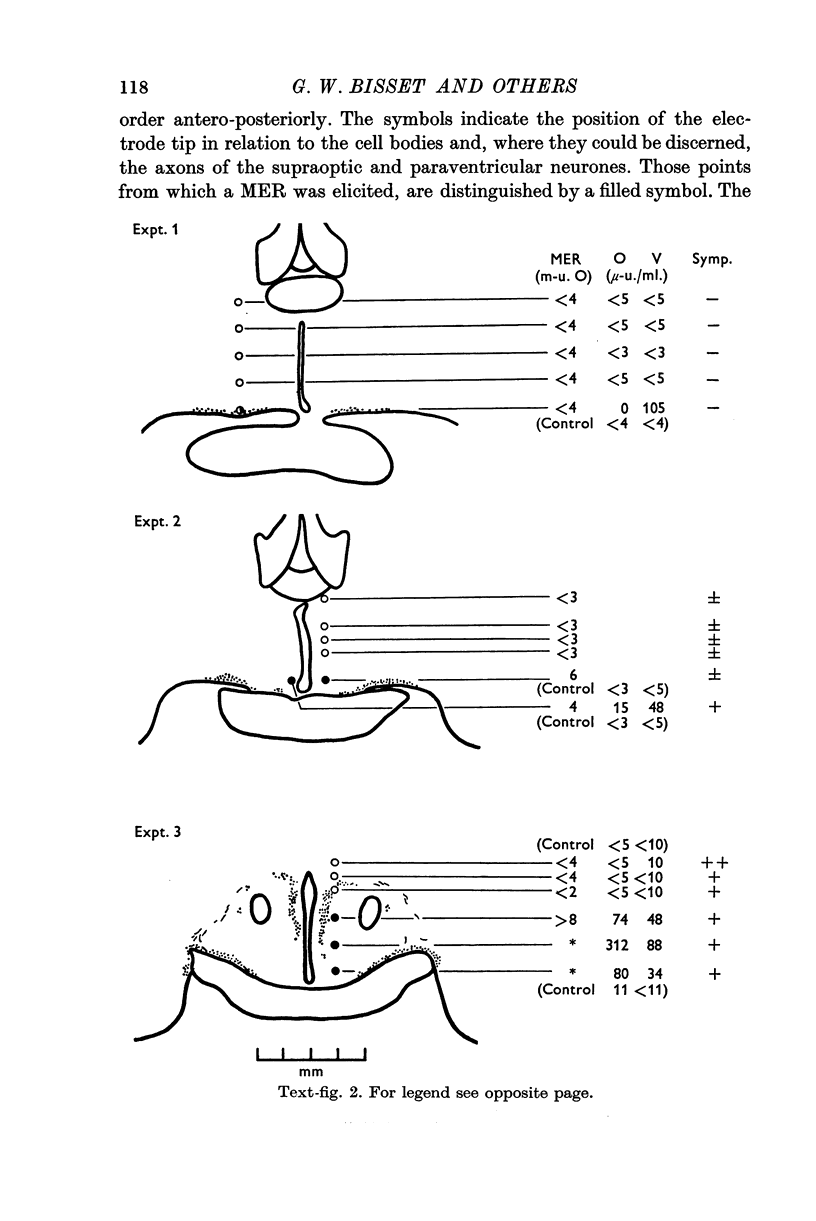
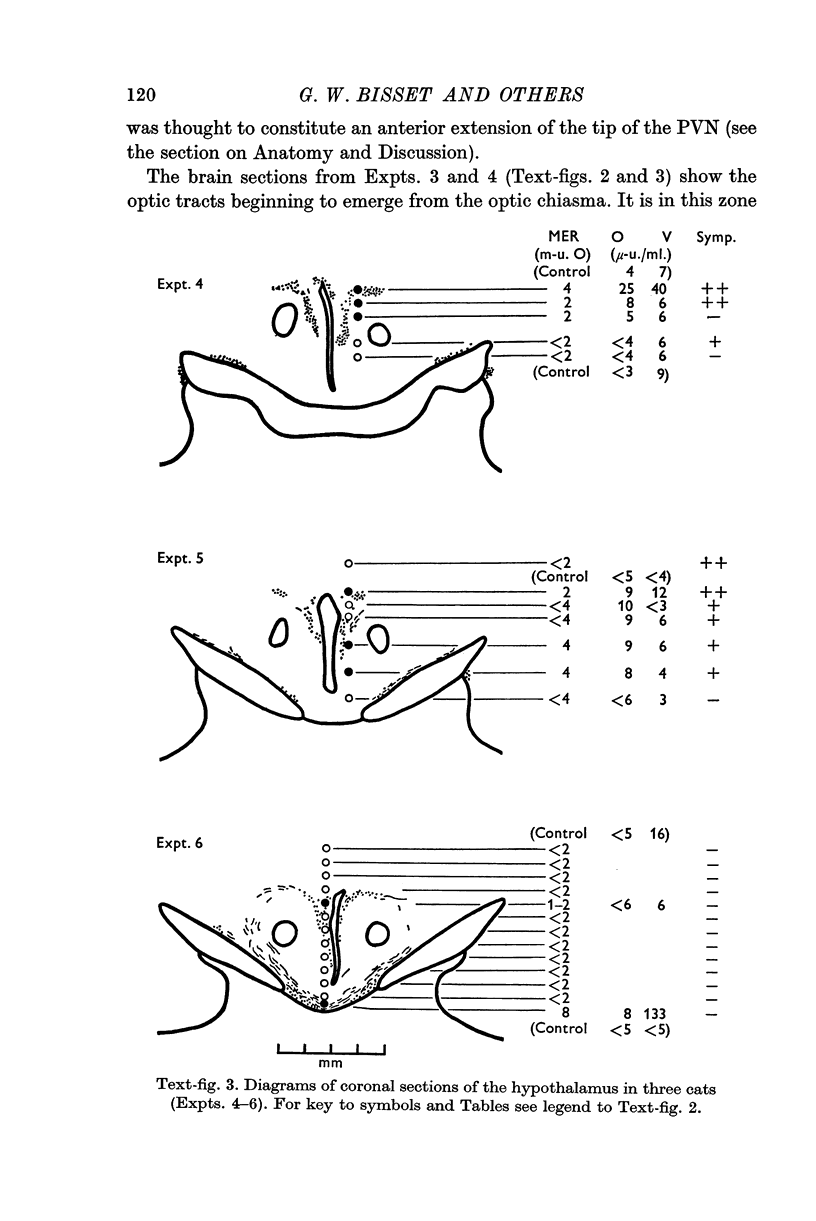
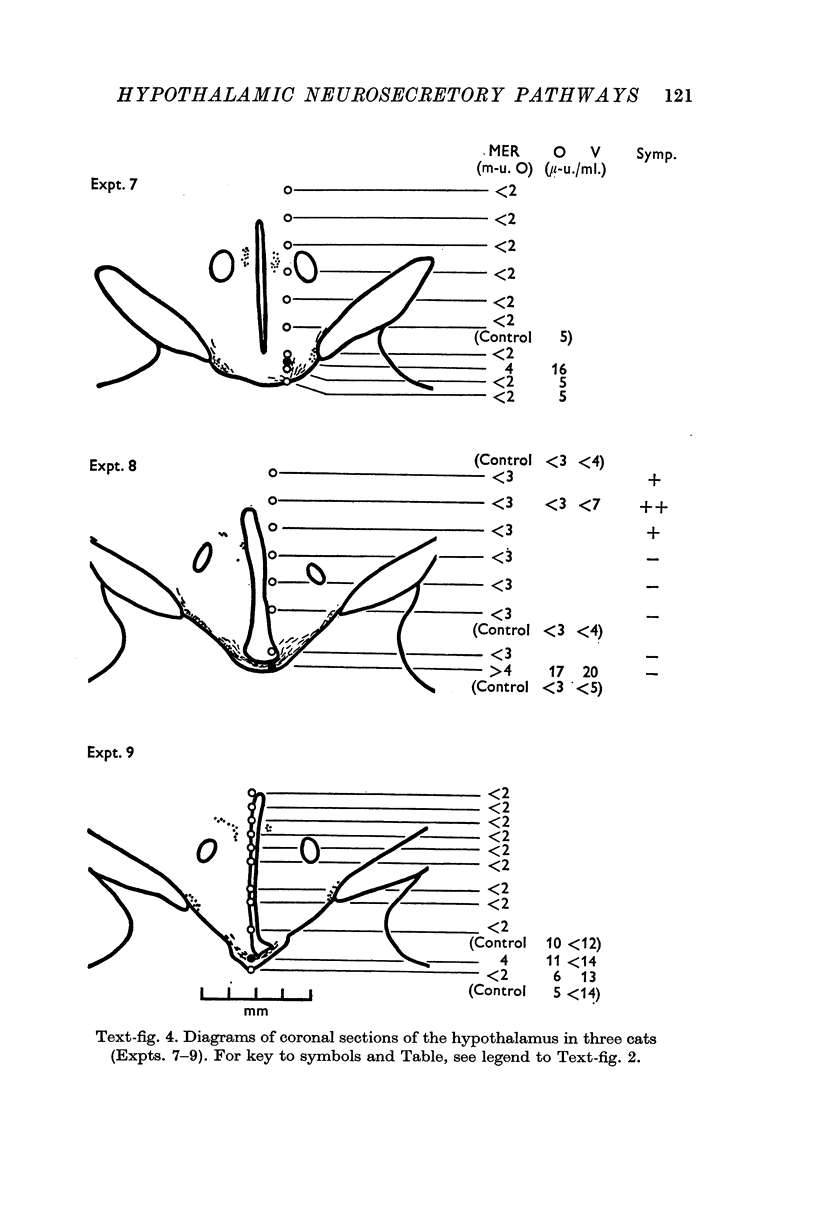
1. The neurones of the supraoptic nucleus (SON) and paraventricular nucleus (PVN) were stimulated electrically in lactating cats under chloralose anaesthesia. Milk-ejection responses were used to monitor the release of oxytocin and vasopressin and both hormones were assayed in samples of blood collected during stimulation. The position of the tip of the stimulating electrode was confirmed from brain sections stained selectively for cystine-rich neurosecretory material.
2. A previous finding that stimulation of the SON in the cat releases vasopressin without oxytocin was confirmed.
3. Stimulation of the PVN caused both hormones to be released. The ratio of their concentrations in blood was variable; this suggests release from separate neurones.
4. Both hormones were also released on stimulation of the median eminence but not of the zone lying vertically between this structure and the PVN. No neurosecretory material was detected in this zone. These findings argue against the existence of a direct or medial paraventriculo-hypophysial pathway running downwards along the wall of the third ventricle.
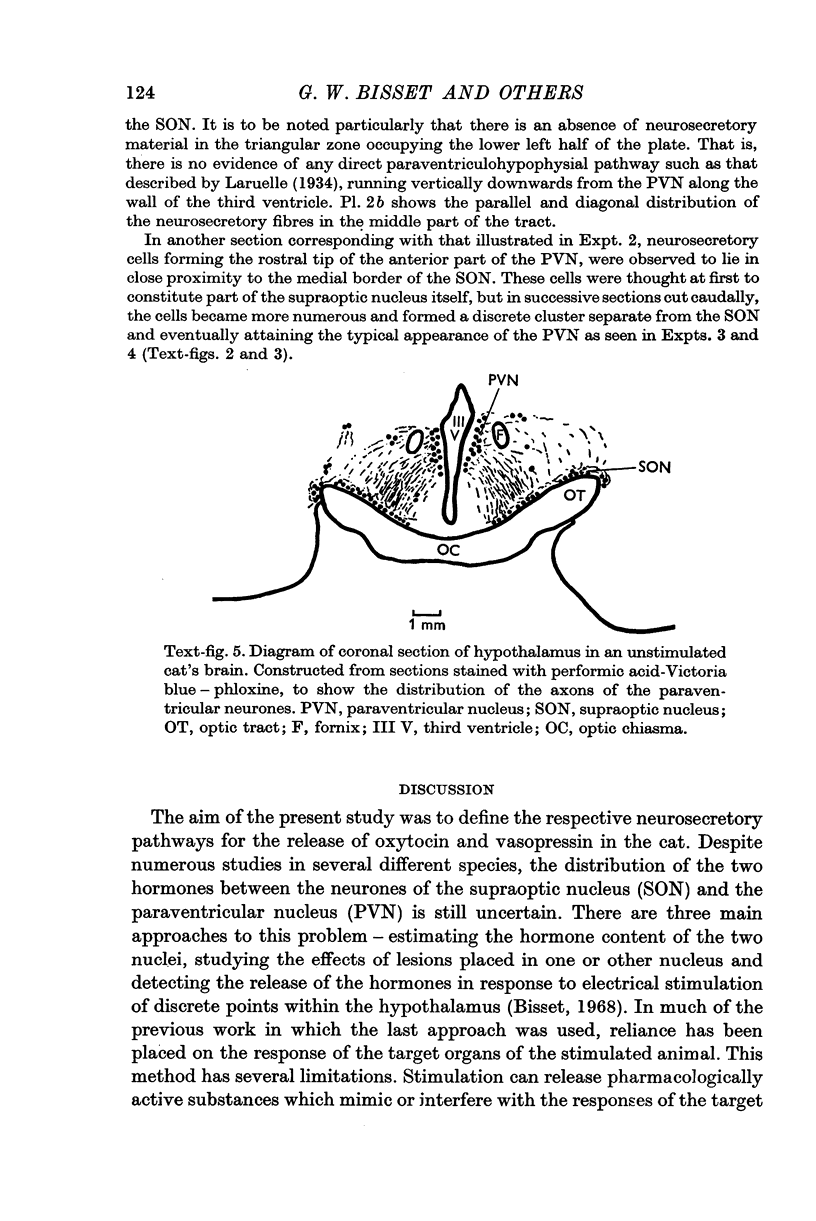
5. Study of sections from unstimulated brains confirmed that the tractus paraventricularis cinereus of Greving which runs ventro-laterally from the PVN towards the SON, represents the principal efferent pathway for neurosecretory fibres from the PVN.
6. The results are discussed in relation to the problem of the independent release of oxytocin and vasopressin in response to physiological stimulation of the neurohypophysis.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. W., SLOPER J. C. The hypothalamic elaboration of posterior pituitary principles in man, the rat and dog; histochemical evidence derived from a performic acid-Alcian blue reaction for cystine. J Endocrinol. 1956 Apr;13(3):221–228. doi: 10.1677/joe.0.0130221. [DOI] [PubMed] [Google Scholar]
- ANDERSSON B., MCCANN S. M. Drinking, antidiuresis and milk ejection from electrical stimulation within the hypothalamus of the goat. Acta Physiol Scand. 1955 Dec 31;35(2):191–201. doi: 10.1111/j.1748-1716.1955.tb01277.x. [DOI] [PubMed] [Google Scholar]
- ANDERSSON B. The effect and localization of electrical stimulation of certain parts of the brain stem in sheep and goats. Acta Physiol Scand. 1951 Jul 30;23(1):8–23. doi: 10.1111/j.1748-1716.1951.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Aulsebrook L. H., Holland R. C. Central regulation of oxytocin release with and without vasopressin release. Am J Physiol. 1969 Apr;216(4):818–829. doi: 10.1152/ajplegacy.1969.216.4.818. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- BARGMANN W. Ueber die neurosekretorische Verknüpfung von Hypothalamus und Neurophypophyse. Z Zellforsch Mikrosk Anat. 1949;34(5):610–634. [PubMed] [Google Scholar]
- BISSET G. W. Effect of tyrosinase preparations on oxytocin, vasopressin and bradykinin. Br J Pharmacol Chemother. 1962 Apr;18:405–420. doi: 10.1111/j.1476-5381.1962.tb01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J. Blood levels of oxytocin and vasopressin during suckling in the rabbit and the problem of their independent release. J Physiol. 1970 Mar;206(3):711–722. doi: 10.1113/jphysiol.1970.sp009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J., Harris M. C., Lewis G. P., Rocha e Silva R., Jr The assay of milk-ejecting activity in the lactating rat. Br J Pharmacol Chemother. 1967 Nov;31(3):537–549. doi: 10.1111/j.1476-5381.1967.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Krejcí I., Polácek I., Rudinger J. Some pharmacological properties of a synthetic oxytocin analogue [1-N-carbamoyl-hemicystine-2-O-methyltyrosine]-oxytocin (carbamoyl-methyloxytocin), an antagonist to the neurohypophysial hormones. Br J Pharmacol. 1970 Oct;40(2):342–360. doi: 10.1111/j.1476-5381.1970.tb09927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Lewis G. P. The mechanism of the inhibitory action of adrenaline on the mammary gland. Br J Pharmacol Chemother. 1967 Nov;31(3):550–559. doi: 10.1111/j.1476-5381.1967.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J. Synthetic 1-N-carbamylhemicystine-2-O-methyltyrosine-oxytocin (N-carbamyl-O-methyl-oxytocin): a specific antagonist to the actions of oxytocin and vasopressin on the uterus and mammary gland. Nature. 1968 Apr 13;218(5137):197–199. doi: 10.1038/218197b0. [DOI] [PubMed] [Google Scholar]
- Brooks C. M., Ishikawa T., Koizumi K., Lu H. H. Activity of neurones in the paraventricular nucleus of the hypothalamus and its control. J Physiol. 1966 Jan;182(1):217–231. doi: 10.1113/jphysiol.1966.sp007820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS B. A. The motility and reactivity of the oestrogenized rabbit uterus in vivo; with comparative observations on milk ejection. J Endocrinol. 1958 Feb;16(3):237–260. doi: 10.1677/joe.0.0160237. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Silva MR Jr E. An afferent pathway for the selective release of vasopressin in response to carotid occlusion and haemorrhage in the cat. J Physiol. 1967 Aug;191(3):529–542. doi: 10.1113/jphysiol.1967.sp008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E. A method for the assay of very small amounts of antidiuretic activity with a note on the antidiuretic titre of rat's blood. J Physiol. 1953 Oct;122(1):149–157. doi: 10.1113/jphysiol.1953.sp004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG H. S., LIU H. M., WANG S. C. Liberation of antidiuretic hormone following hypothalamic stimulation in the dog. Am J Physiol. 1962 Feb;202:212–216. doi: 10.1152/ajplegacy.1962.202.2.212. [DOI] [PubMed] [Google Scholar]
- FORD D. H., KANTOUNIS S. The localization of neurosecretory structures and pathways in the male albino rabbit. J Comp Neurol. 1957 Aug;108(1):91–107. doi: 10.1002/cne.901080105. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., SMITH M. W. The fate of oxytocin in male and female rats. Br J Pharmacol Chemother. 1959 Sep;14:327–333. doi: 10.1111/j.1476-5381.1959.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar J. Independent release of oxytocin and vasopressin during parturition in the rabbit. J Physiol. 1970 Mar;206(3):723–730. doi: 10.1113/jphysiol.1970.sp009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUVER H., BARRERA E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953 Oct;12(4):400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- KOELLA W. Die Beeinflussung der Harnsekretion durch hypothalamische Reizung. Helv Physiol Pharmacol Acta. 1949;7(4):498–514. [PubMed] [Google Scholar]
- LAQUEUR G. L. Neurosecretory pathways between the hypothalamic paraventricular nucleus and the neurohypophysis. J Comp Neurol. 1954 Dec;101(3):543–563. doi: 10.1002/cne.901010302. [DOI] [PubMed] [Google Scholar]
- NIBBELINK D. W. Paraventricular nuclei, neurohypophysis, and parturition. Am J Physiol. 1961 Jun;200:1229–1232. doi: 10.1152/ajplegacy.1961.200.6.1229. [DOI] [PubMed] [Google Scholar]
- OLIVECRONA H. Paraventricular nucleus and pituitary gland. Acta Physiol Scand Suppl. 1957;40(136):1–178. [PubMed] [Google Scholar]
- Schrier R. W., Verroust P. J., Jones J. J., Fabian M., Lee J., De Wardener H. E. Effect of isotonic saline infusion and acute haemorrhage on plasma oxytocin and vasopressin concentrations in dogs. Clin Sci. 1968 Dec;35(3):433–443. [PubMed] [Google Scholar]
- Smyth D. G. Acetylation of amino and tyrosine hydroxyl groups. Preparation of inhibitors of oxytocin with no intrinsic activity on the isolated uterus. J Biol Chem. 1967 Apr 10;242(7):1592–1598. [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. Preferential release of oxytocin from the neurohypophysis after electrical stimulation of the afferent path of the milk-ejection reflex in the brain of the guinea-pig. J Endocrinol. 1968 Feb;40(2):205–214. doi: 10.1677/joe.0.0400205. [DOI] [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. The afferent path of the milk-ejection reflex in the brain of the guinea-pig. J Endocrinol. 1967 Jul;38(3):337–349. doi: 10.1677/joe.0.0380337. [DOI] [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. The afferent path of the milk-ejection reflex in the brain of the rabbit. J Endocrinol. 1969 Apr;43(4):663–671. doi: 10.1677/joe.0.0430663. [DOI] [PubMed] [Google Scholar]




