Abstract
1. A study has been made of the T-system and sarcoplasmic reticulum (SR) in slow muscle fibres of the frog, Rana temporaria.
2. The size of the T-system was measured by an autoradiographic method, using tritium-labelled albumin as a marker. Its volume, expressed as a fraction of that of the fibre, was found to be 1·8 × 10-3, as compared with a figure of 3·9 × 10-3 for the T-system in a twitch fibre.
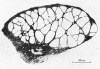
3. The spatial distribution of the T-tubules, and their association with the SR, was studied with the electron microscope, employing ferritin and the enzyme peroxidase as markers. The observations show (a) the tubules form a three dimensional, rather than transverse, network and (b) the area of triadic (and diadic) contact with the SR is 5-10 × smaller than in a twitch fibre.
4. The possibility that the T-system and SR of the slow fibre participate in linking membrane excitation with contraction is discussed in the light of these findings.
Full text
PDF












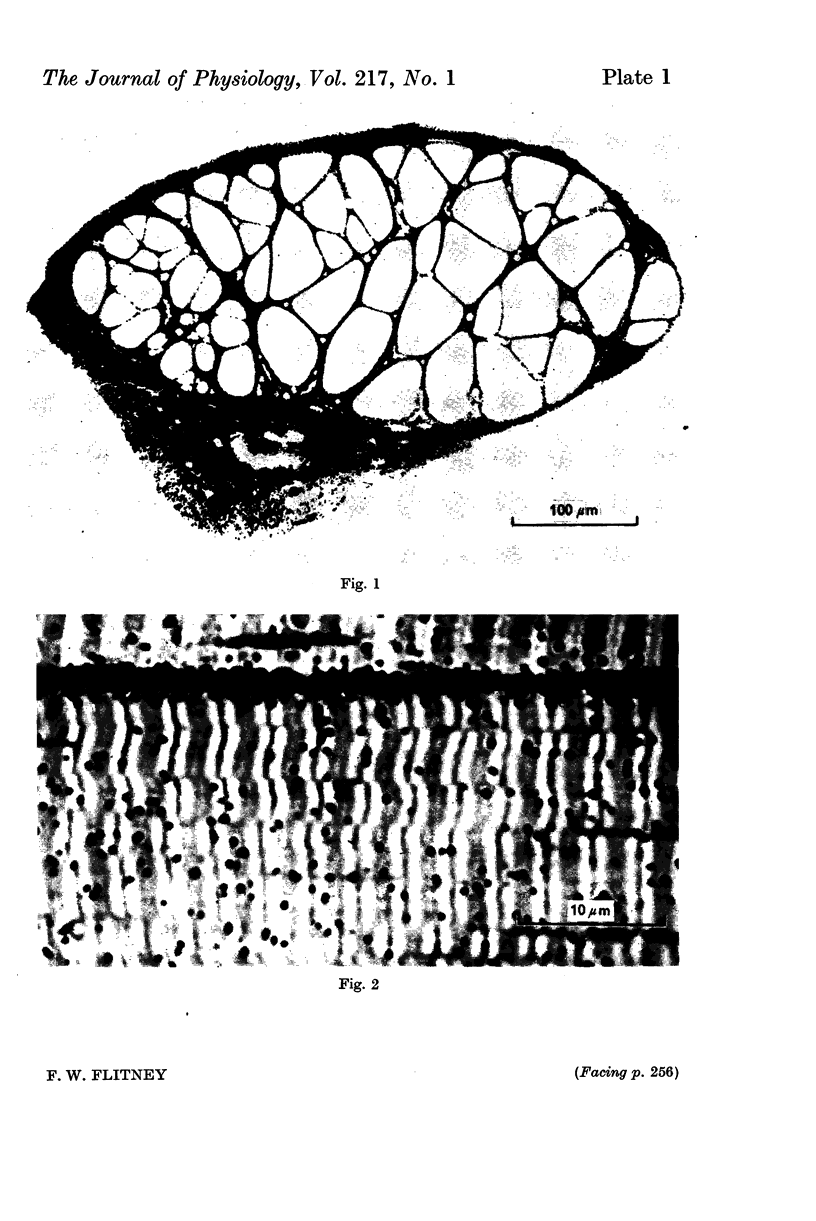




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURKE W., GINSBORG B. L. The action of the neuromuscular transmitter on the slow fibre membrane. J Physiol. 1956 Jun 28;132(3):599–610. doi: 10.1113/jphysiol.1956.sp005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Lopez E., Reuben J. P., Grundfest H. The relationship between myofilament packing density and sarcomere length in frog striated muscle. J Cell Biol. 1967 May 1;33(2):255–263. doi: 10.1083/jcb.33.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSEN F., KNAPPEIS G. G., BUCHTHAL F. Ultrastructure of the resting and contracted striated muscle fiber at different degrees of stretch. J Biophys Biochem Cytol. 1961 Oct;11:95–117. doi: 10.1083/jcb.11.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J., Tice L. W. Calcium activation of frog slow muscle fibres. J Physiol. 1967 Jan;188(2):261–271. doi: 10.1113/jphysiol.1967.sp008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W. K., Irwin R. L. A histochemical-physiological correlation of frog skeletal muscle fibers. Am J Physiol. 1967 Aug;213(2):511–518. doi: 10.1152/ajplegacy.1967.213.2.511. [DOI] [PubMed] [Google Scholar]
- FEWSTER M. E., HALL D. A. Application of buffered solvent systems to the detection of aromatic acids by paper partition chromatography. Nature. 1951 Jul 14;168(4263):78–79. doi: 10.1038/168078a0. [DOI] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. SARCOLEMMAL INVAGINATIONS CONSTITUTING THE T SYSTEM IN FISH MUSCLE FIBERS. J Cell Biol. 1964 Sep;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C., PEACHEY L. D. THE RELATION BETWEEN THE LATE AFTER-POTENTIAL AND THE SIZE OF THE TRANSVERSE TUBULAR SYSTEM OF FROG MUSCLE. J Gen Physiol. 1964 Nov;48:235–263. doi: 10.1085/jgp.48.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K., Smith I. C. The mechanical and thermal properties of frog slow muscle fibres. J Physiol. 1971 Mar;213(3):617–631. doi: 10.1113/jphysiol.1971.sp009404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. Autoradiographic localization of adenine nucleotide in frog's striated muscle. J Physiol. 1959 Jan 28;145(1):132–174. doi: 10.1113/jphysiol.1959.sp006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. THE SPACE ACCESSIBLE TO ALBUMIN WITHIN THE STRIATED MUSCLE FIBRE OF THE TOAD. J Physiol. 1964 Dec;175:275–294. doi: 10.1113/jphysiol.1964.sp007517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Local activation of muscle. Ann N Y Acad Sci. 1959 Aug 28;81:446–452. doi: 10.1111/j.1749-6632.1959.tb49326.x. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Hess A. The structure of vertebrate slow and twitch muscle fibers. Invest Ophthalmol. 1967 Jun;6(3):217–228. [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol. 1953 Aug;121(2):318–340. doi: 10.1113/jphysiol.1953.sp004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. E. The fine structure of skeletal muscle triad junctions. J Ultrastruct Res. 1969 Oct;29(1):37–49. doi: 10.1016/s0022-5320(69)80054-7. [DOI] [PubMed] [Google Scholar]
- Krolenko S. A. Changes in the T-system of muscle fibres under the influence of influx and efflux of glycerol. Nature. 1969 Mar 8;221(5184):966–968. doi: 10.1038/221966a0. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Nakajima Y., Peachey L. D. Speed of repolarization and morphology of glycerol-treated muscle fibres. J Physiol. 1969 Feb;200(2):115P–116P. [PubMed] [Google Scholar]
- Nasledov G. A., Zachar J., Zacharová D. The ionic requirements for the development of contracture in isolated slow muscle fibres of the frog. Physiol Bohemoslov. 1966;15(4):293–306. [PubMed] [Google Scholar]
- ORKAND R. K. A further study of electrical responses in slow and twitch muscle fibres of the frog. J Physiol. 1963 Jun;167:181–191. doi: 10.1113/jphysiol.1963.sp007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUSCHINGER P., BRECHT K. Influence of calcium on the potassium-contracture of 'slow' and 'fast' skeletal muscle fibres of the frog. Nature. 1961 Feb 18;189:583–584. doi: 10.1038/189583a0. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D., HUXLEY A. F. Structural identification of twitch and slow striated muscle fibers of the frog. J Cell Biol. 1962 Apr;13:177–180. doi: 10.1083/jcb.13.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. A comparison of the fine structures of frog slow and twitch muscle fibers. J Cell Biol. 1965 Aug;26(2):477–497. doi: 10.1083/jcb.26.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. Structure of the sarcoplasmic reticulum in vertebrate muscle. Br Med Bull. 1968 May;24(2):170–173. doi: 10.1093/oxfordjournals.bmb.a070621. [DOI] [PubMed] [Google Scholar]
- Peachey L. D., Schild R. F. The distribution of the T-system along the sarcomeres of frog and toad sartorius muscles. J Physiol. 1968 Jan;194(1):249–258. doi: 10.1113/jphysiol.1968.sp008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N. Rapid uptake of tritiated antigen by mouse eosinophils. Nature. 1966 Apr 16;210(5033):266–269. doi: 10.1038/210266a0. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. Persistence of excitation contraction coupling in "slow" muscle fibres after a treatment that destroys transverse tubules in "twitch" fibres. Nature. 1968 May 18;218(5142):681–682. doi: 10.1038/218681a0. [DOI] [PubMed] [Google Scholar]