Abstract
1. Catheters were inserted into the maternal and foetal vasculatures of ten ewes, 100-139 days pregnant, and daily samples of uterine and umbilical blood and maternal jugular vein blood were taken for periods of 5-27 days after operation.
2. Catheters were inserted into the fluid sacs of nineteen foetuses, 60-97 days post-conception, and daily samples were withdrawn for up to 90 days from amniotic sacs (eleven foetuses) and for up to 70 days from allantoic sacs (eight foetuses). Maternal jugular plasma was obtained 3 times weekly and an approximation from its composition to that of uterine and umbilical plasma was made using results from the ewes and foetuses with vascular catheters.
3. The pH, osmolality, [Na+], [K+], [Cl-], [urea] and [amino acid] of all samples were measured.
4. The nutritional status of all ewes was monitored throughout pregnancy. Most lambs were born naturally at ∼ 147 days post-conception and their subsequent progress was observed.
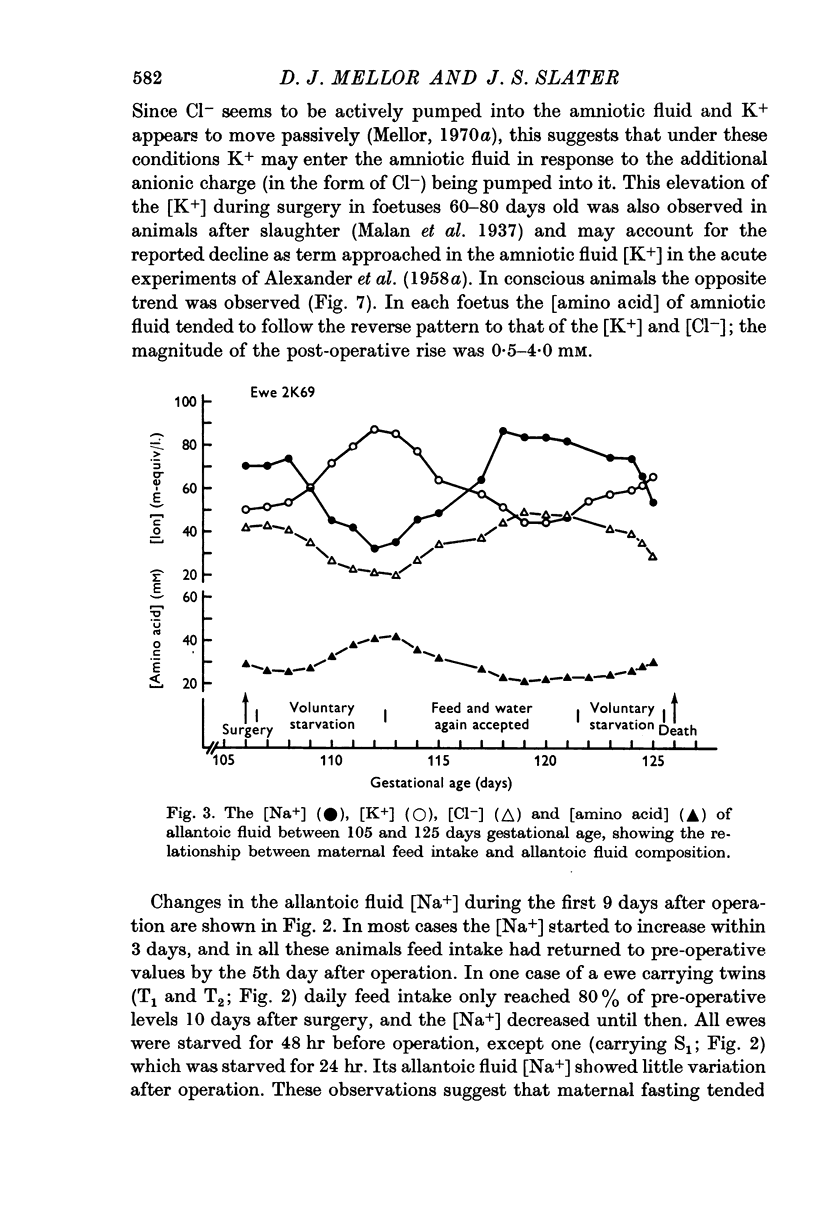
5. Results at operation and from acute experiments were compared with those from conscious ewes and foetuses of the same gestational age to assess the nature and extent of the influence of the operative procedures on foetal fluid composition. The composition of amniotic fluid was influenced mainly by the anaesthetic and surgical procedures while that of allantoic fluid was affected largely by starvation of the ewe.
6. Changes during recovery from operation were followed and indicated that maternal and foetal plasma required about 3 days and the foetal fluids up to 7 days before stability of composition was achieved.
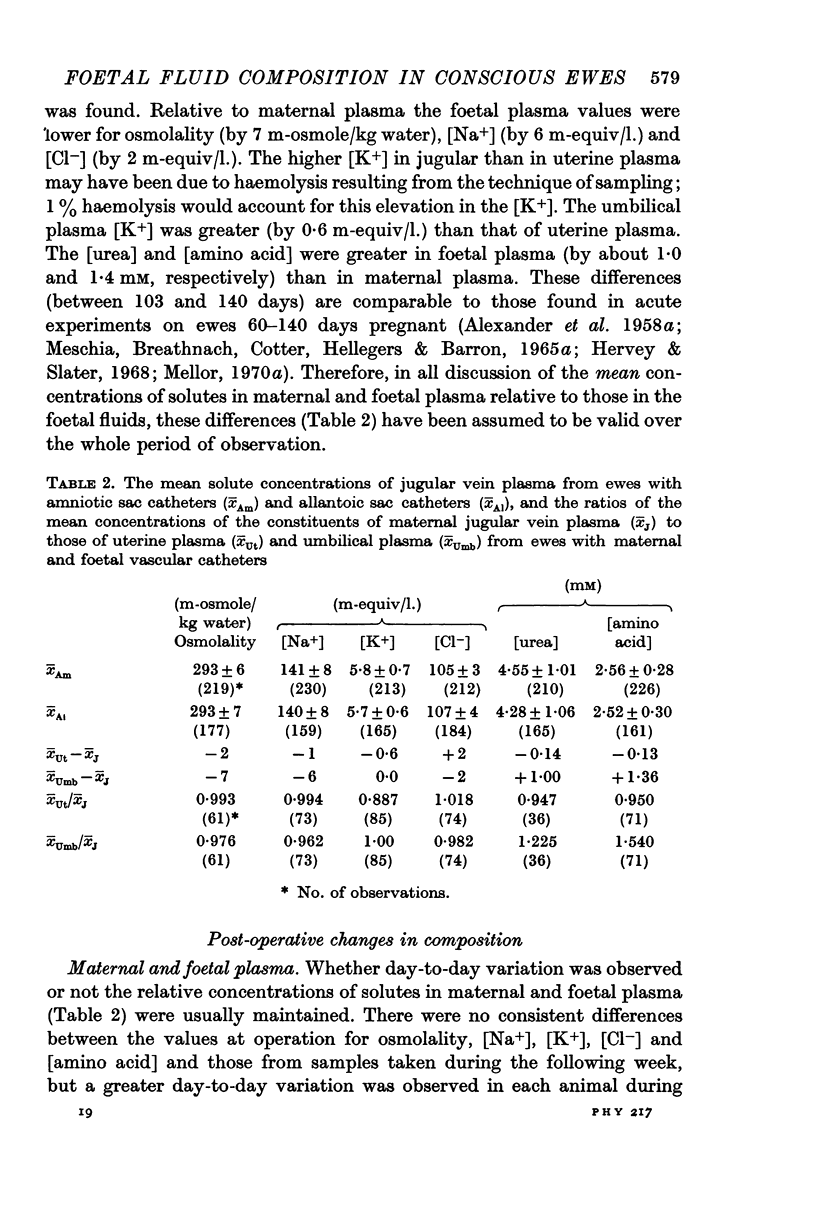
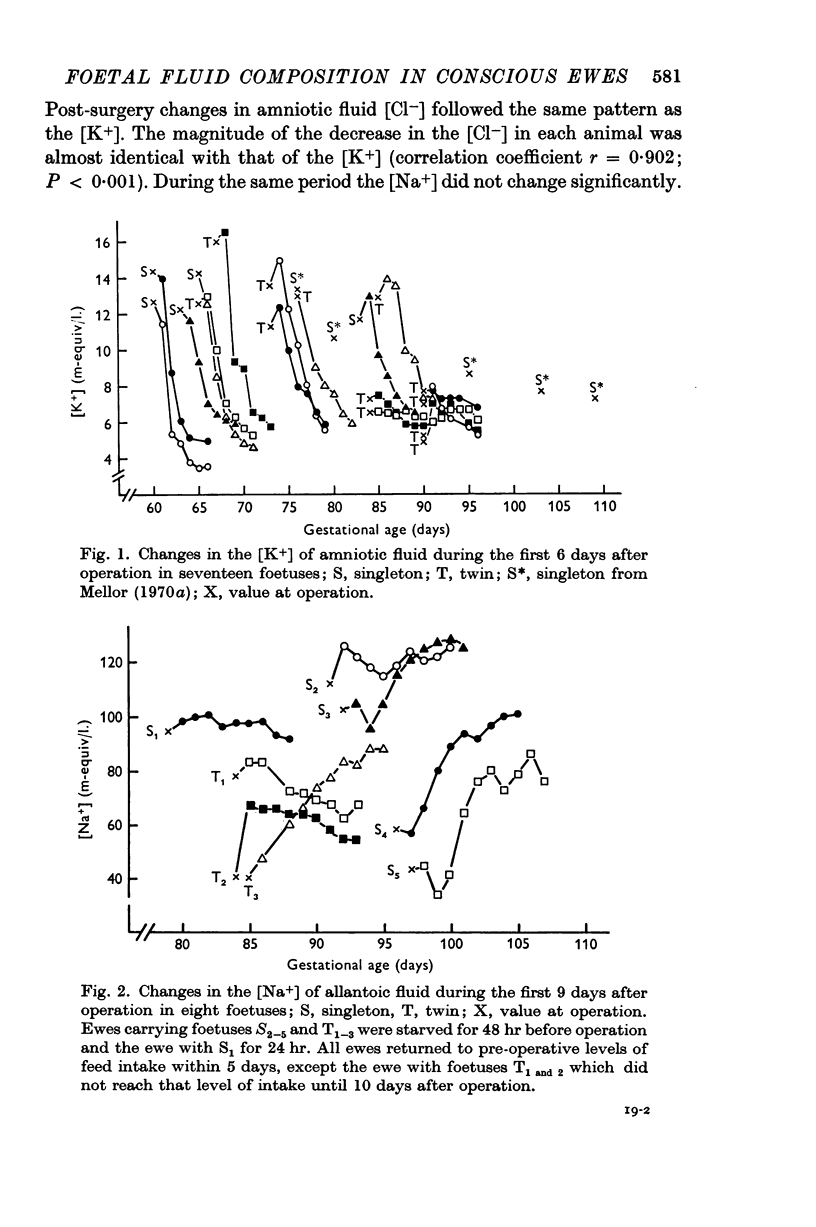
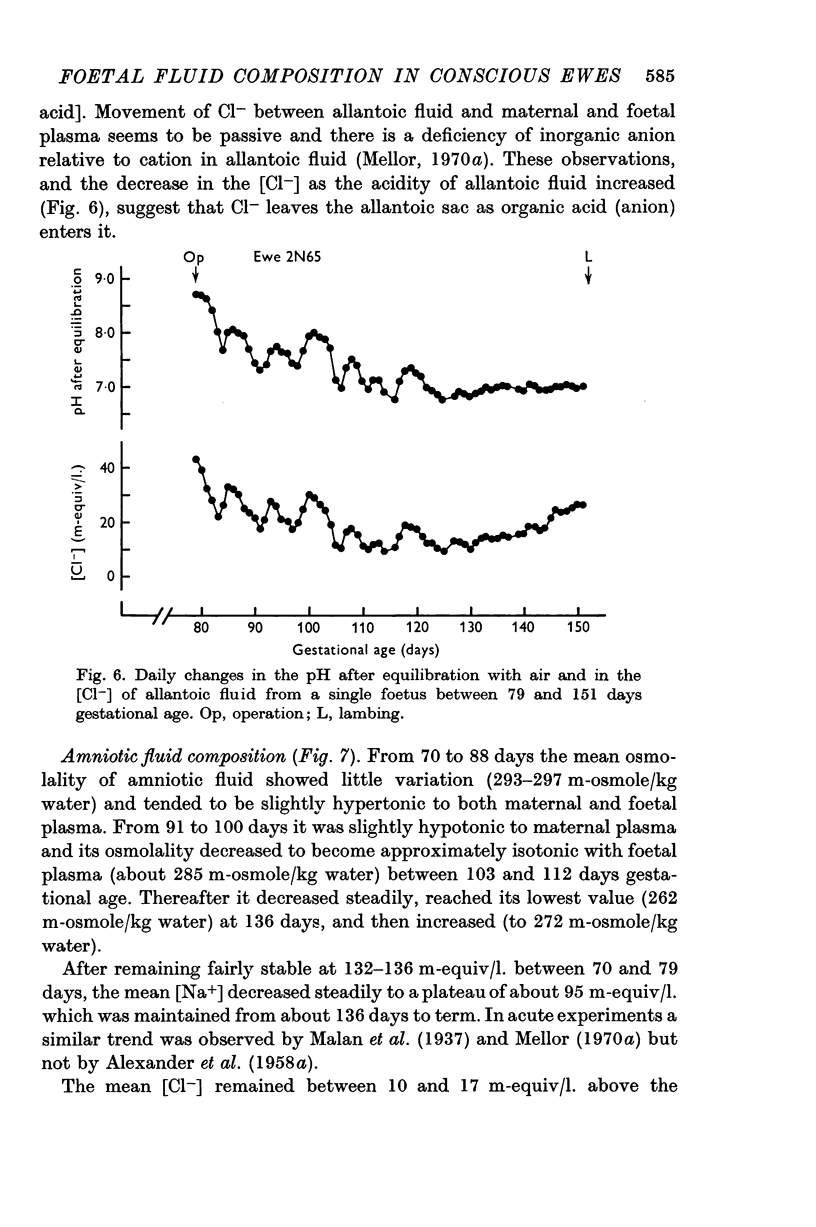
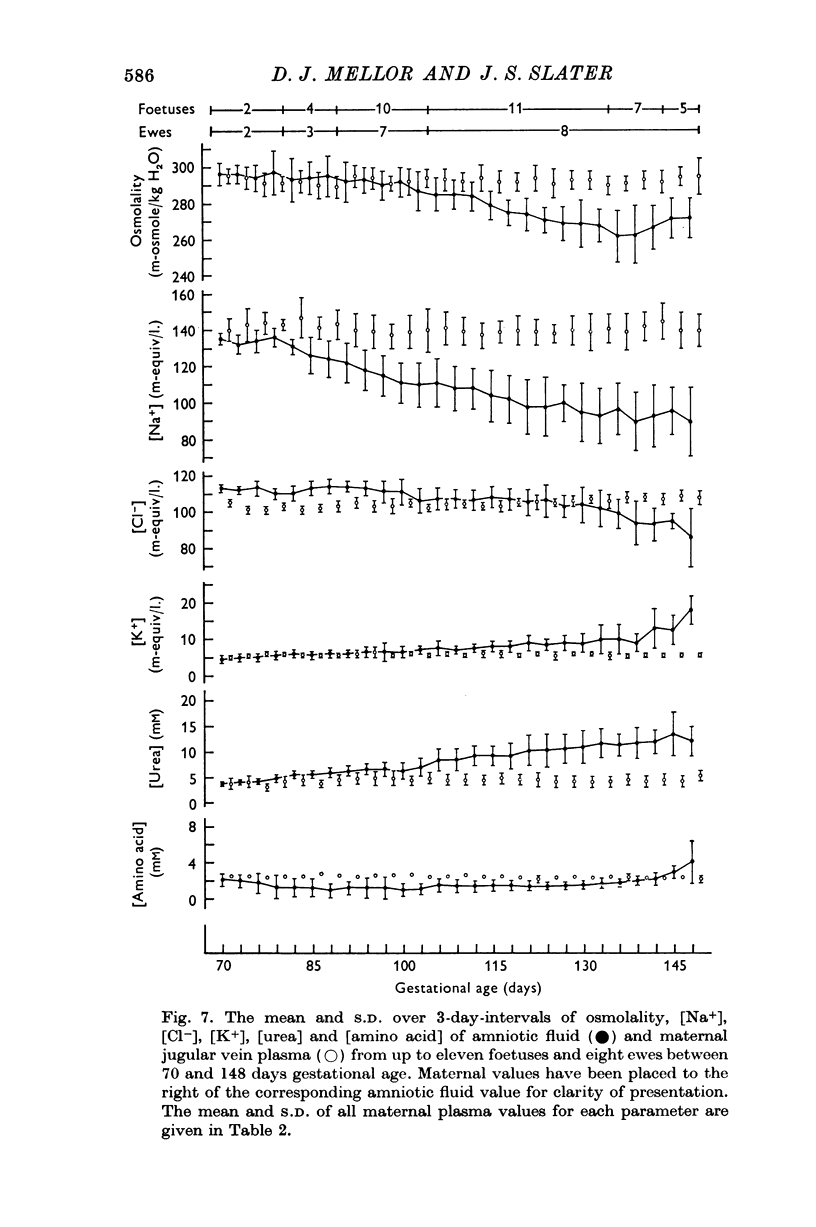
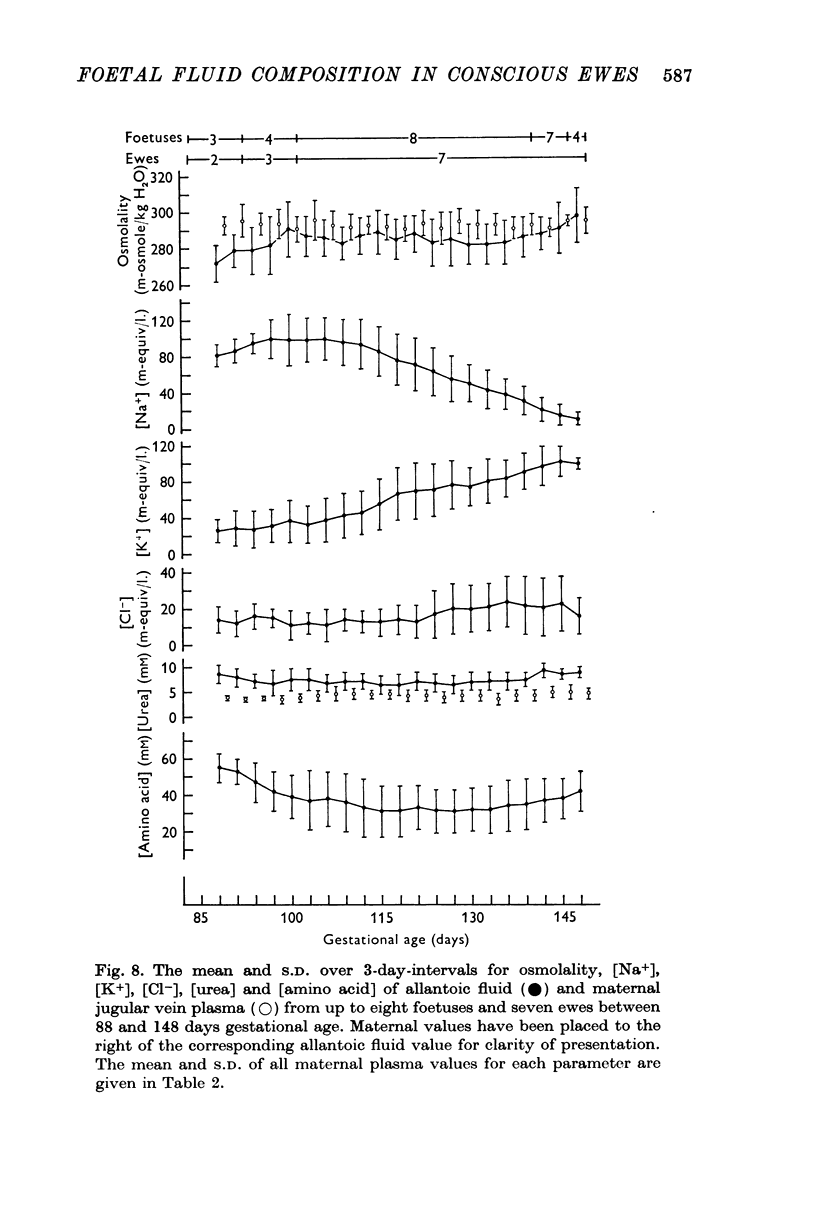
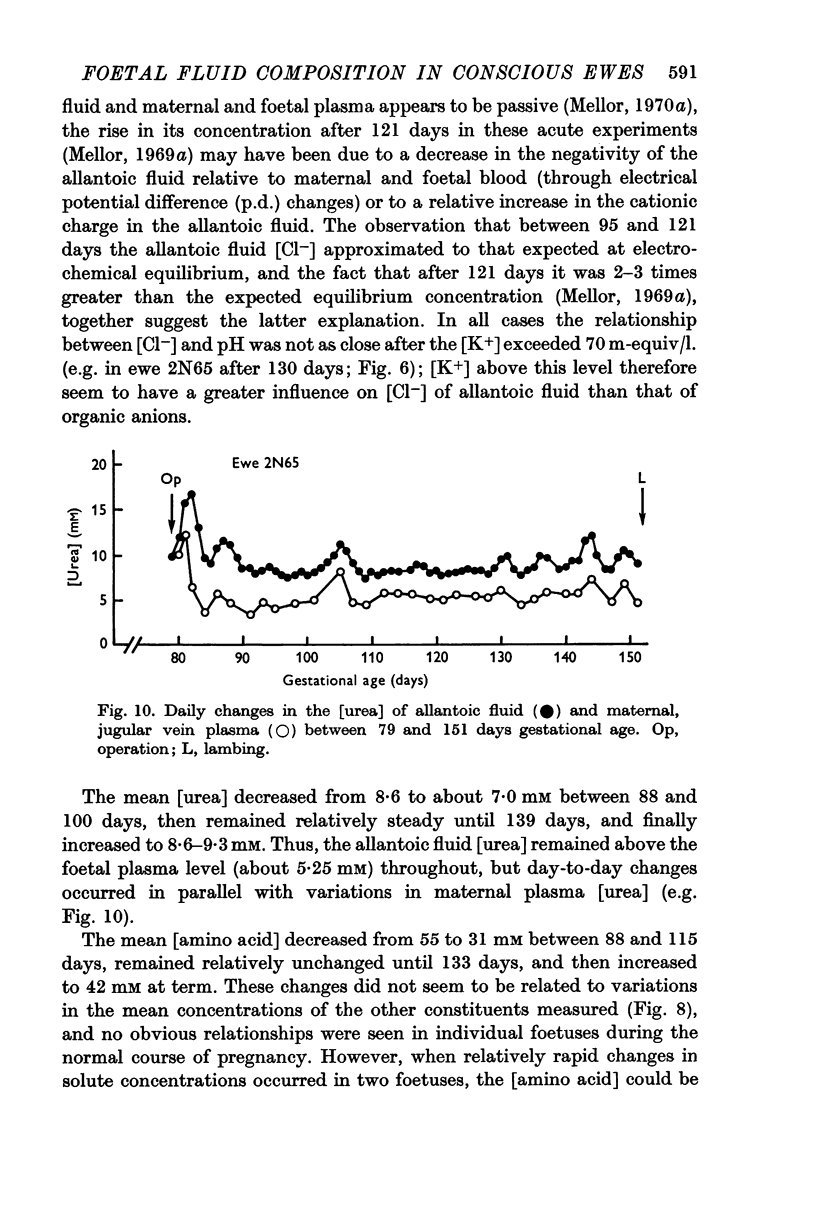
7. After recovery from operation, daily changes in the composition of each foetal fluid showed the same general pattern in all foetuses, but the absolute values of constituents sometimes showed large differences.
8. It is suggested that flow of foetal urine into the amniotic sac increased from 80 days gestational age, that urine flow into the allantoic sac decreased until about 100 days but did not cease thereafter, and that relative to foetal urine the influence of foetal pulmonary fluid on amniotic fluid composition was not great.
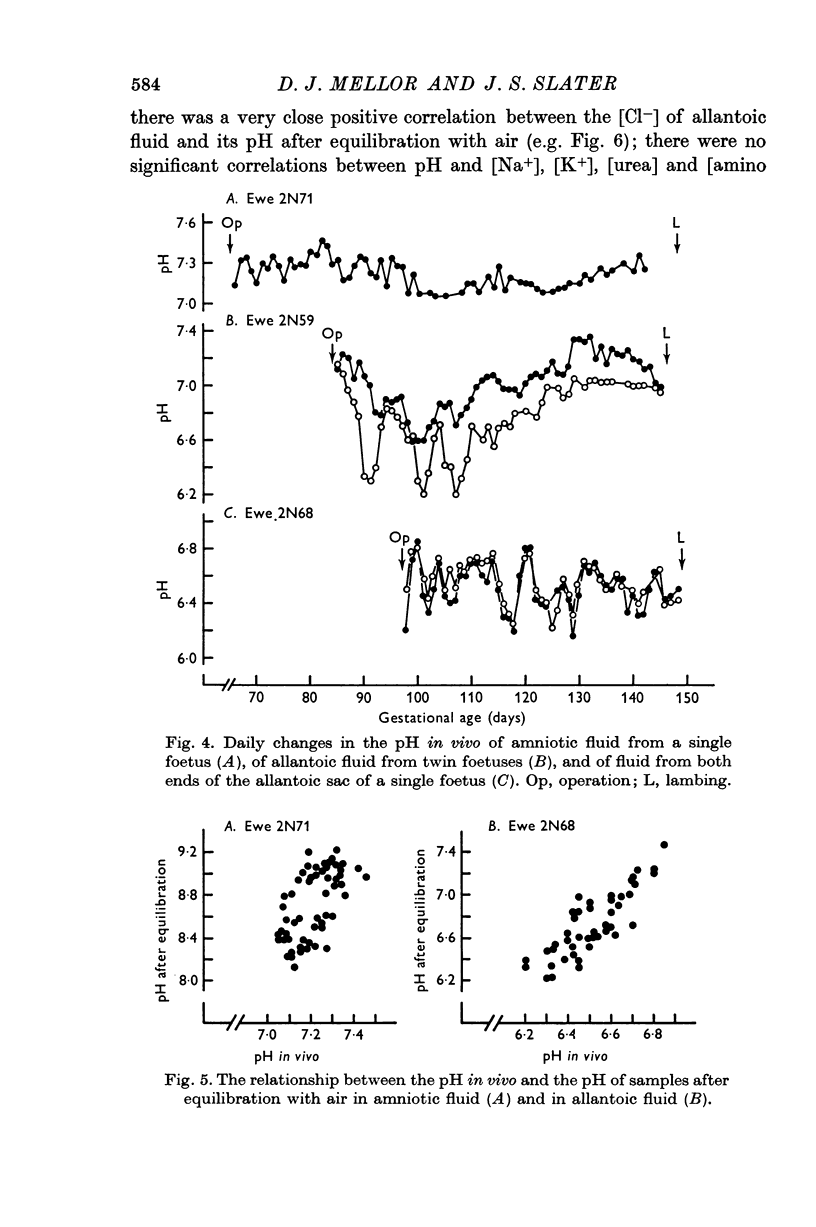
9. A relative impermeability of the amnion appeared to be a major factor influencing amniotic fluid composition, whereas pumping mechanisms in the chorioallantois seem to have been responsible largely for changes in the composition of allantoic fluid.
10. The quantity of solute relative to that of water within each sac appears to be a major determinant of changes in foetal fluid volumes.
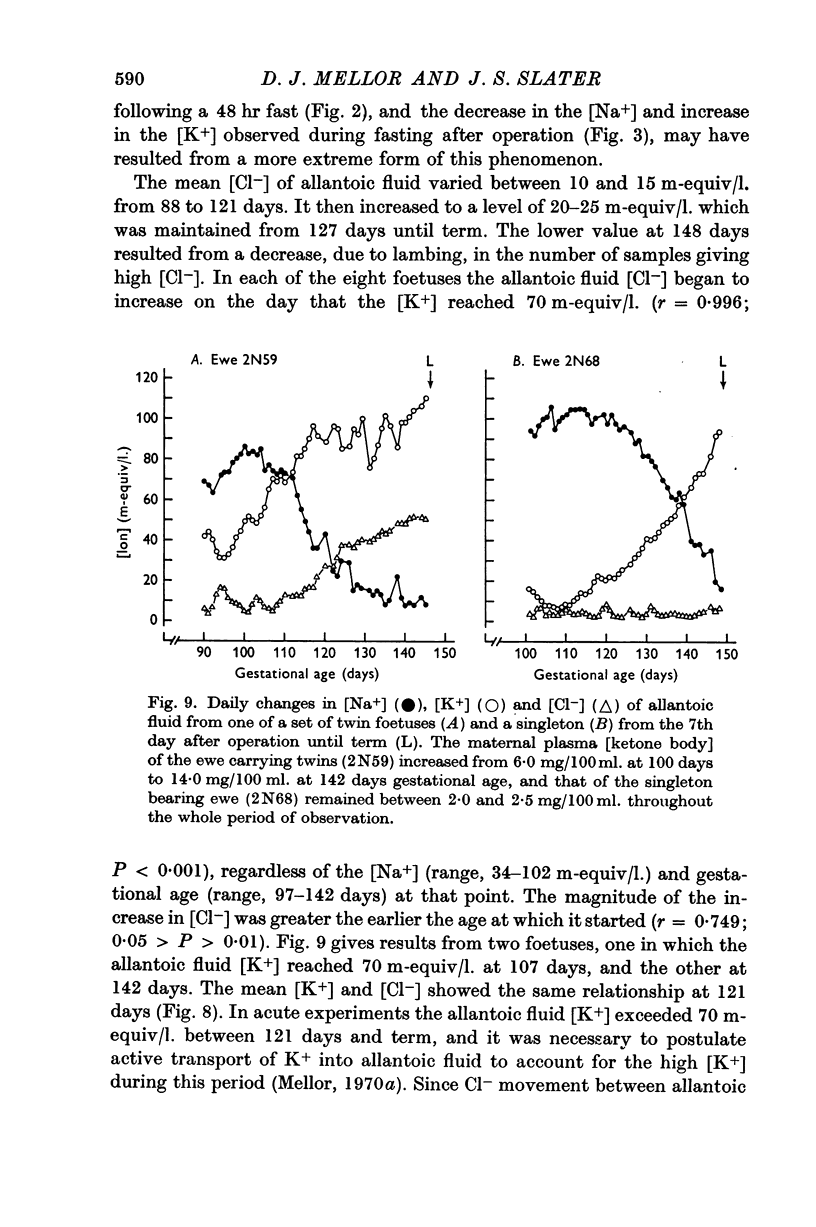
11. Changes in the [Na+] and [K+] of allantoic fluid during the normal course of pregnancy were consistent with an increasing action of mineralo-corticoids on pumping mechanisms in the chorioallantois. Similar but more rapid changes seemed to be associated with acute and chronic episodes of maternal hypoglycaemia. Under these circumstances foetal hypoglycaemia may effect a relative increase in the secretion of foetal corticosteroids having an action on the chorioallantois.
12. The results from this study demonstrate clearly the value of using chronically catheterized animals, and it is suggested that their use in physiological studies on the conceptus must eventually supersede that of acute, anaesthetized preparations.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER D. P., NIXON D. A., WIDDAS W. F., WOHLZOGEN F. X. Gestational variations in the composition of the foetal fluids and foetal urine in the sheep. J Physiol. 1958 Jan 23;140(1):1–13. doi: 10.1113/jphysiol.1958.sp005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER D. P., NIXON D. A., WIDDAS W. F., WOHLZOGEN F. X. Renal function in the sheep foetus. J Physiol. 1958 Jan 23;140(1):14–22. doi: 10.1113/jphysiol.1958.sp005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams F. H., Desilets D. T., Towers B. Control of flow of fetal lung fluid at the laryngeal outlet. Respir Physiol. 1967 May;2(3):302–309. doi: 10.1016/0034-5687(67)90035-7. [DOI] [PubMed] [Google Scholar]
- Adams F., Desilets D. T., Towers B. Physiology of the fetal larynx and lung. Ann Otol Rhinol Laryngol. 1967 Oct;76(4):735–743. doi: 10.1177/000348946707600401. [DOI] [PubMed] [Google Scholar]
- Adamson T. M., Boyd R. D., Platt H. S., Strang L. B. Composition of alveolar liquid in the foetal lamb. J Physiol. 1969 Sep;204(1):159–168. doi: 10.1113/jphysiol.1969.sp008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., Forsling M. L., Nixon D. A., Ratcliffe J. G. The concentrations of adrenocorticotrophin, vasopressin and oxytocin in the foetal and maternal plasma of the sheep in the latter half of gestation. J Endocrinol. 1971 Jan;49(1):179–180. doi: 10.1677/joe.0.0490179. [DOI] [PubMed] [Google Scholar]
- Alexander G., Williams D. Hormonal control of amniotic and allantoic fluid volume in ovariectomized sheep. J Endocrinol. 1968 Aug;41(4):477–485. doi: 10.1677/joe.0.0410477. [DOI] [PubMed] [Google Scholar]
- Bailey R. E., Ford H. C. The effect of heparin on sodium conservation and on the plasma concentration, the metabolic clearance and the secretion and excretion rates of aldosterone in normal subjects. Acta Endocrinol (Copenh) 1969 Feb;60(2):249–264. doi: 10.1530/acta.0.0600249. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Hinks N. T. Micro-determination of corticosteroids in ovine peripheral plasma: effects of venipuncture, corticotrophin, insulin and glucose. J Endocrinol. 1969 Jul;44(3):387–403. doi: 10.1677/joe.0.0440387. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Mills S. C., Reid R. L. The influence of cortisol on glucose utilization in sheep. Metabolism. 1966 Oct;15(10):922–932. doi: 10.1016/0026-0495(66)90163-6. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Oxborrow T. J., Smith I. D., Thorburn G. D. The concentration of progesterone in the peripheral plasma of the pregnant ewe. J Endocrinol. 1969 Nov;45(3):449–457. doi: 10.1677/joe.0.0450449. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol. 1969 Jun;44(2):285–286. doi: 10.1677/joe.0.0440285. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D., Wallace A. L. The plasma growth hormone concentration of the foetal lamb. J Endocrinol. 1970 Oct;48(2):251–263. doi: 10.1677/joe.0.0480251. [DOI] [PubMed] [Google Scholar]
- Challis J. R. Sharp increase in free circulating oestrogens immediately before parturition in sheep. Nature. 1971 Jan 15;229(5281):208–208. doi: 10.1038/229208a0. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Nathanielsz P. W., Paisey R. B., Silver M. Cortisol turnover in the sheep foetus immediately prior to parturition. J Physiol. 1970 Sep;210(2):141P–142P. [PubMed] [Google Scholar]
- Comline R. S., Nathanielsz P. W., Silver M. Passage of thyroxine across the placenta in the foetal sheep. J Physiol. 1970 Mar;207(1):3P–4P. [PubMed] [Google Scholar]
- Comline R. S., Silver M. Daily changes in foetal and maternal blood of conscious pregnant ewes, with catheters in umbilical and uterine vessels. J Physiol. 1970 Aug;209(3):567–586. doi: 10.1113/jphysiol.1970.sp009180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay J. K., Cox R. I. Oestrogens in the plasma of the sheep foetus. J Endocrinol. 1970 Feb;46(2):281–282. doi: 10.1677/joe.0.0460281. [DOI] [PubMed] [Google Scholar]
- JONES I. C., JARRETT I. G., VINSON G. P., POTTER K. ADRENOCORTICOSTEROID PRODUCTION OF FOETAL SHEEP NEAR TERM. J Endocrinol. 1964 May;29:211–212. doi: 10.1677/joe.0.0290211. [DOI] [PubMed] [Google Scholar]
- LINDNER H. R. Blood cortisol in the sheep: normal concentration and changes in ketosis of pregnancy. Nature. 1959 Nov 21;184(Suppl 21):1645–1646. doi: 10.1038/1841645a0. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol. 1968 Oct;42(2):323–329. doi: 10.1677/joe.0.0420323. [DOI] [PubMed] [Google Scholar]
- MESCHIA G., BREATHNACH C. S., COTTER J. R., HELLEGERS A., BARRON D. H. THE DIFFUSIBILITY OF UREA ACROSS THE SHEEP PLACENTA IN THE LAST 2 MONTHS OF GESTATION. Q J Exp Physiol Cogn Med Sci. 1965 Jan;50:23–41. doi: 10.1113/expphysiol.1965.sp001767. [DOI] [PubMed] [Google Scholar]
- MESCHIA G., COTTER J. R., BREATHNACH C. S., BARRON D. H. THE HEMOGLOBIN, OXYGEN, CARBON DIOXIDE AND HYDROGEN ION CONCENTRATIONS IN THE UMBILICAL BLOODS OF SHEEP AND GOATS AS SAMPLED VIA INDWELLING PLASTIC CATHETERS. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:185–195. doi: 10.1113/expphysiol.1965.sp001780. [DOI] [PubMed] [Google Scholar]
- Makowski E. L., Meschia G., Droegemueller W., Battaglia F. C. Measurement of umbilical arterial blood flow to the sheep placenta and fetus in utero. Distribution to cotyledons and the intercotyledonary chorion. Circ Res. 1968 Nov;23(5):623–631. doi: 10.1161/01.res.23.5.623. [DOI] [PubMed] [Google Scholar]
- McDOUGALL E. I. The composition of foetal fluids of sheep at different stages of gestation. Biochem J. 1949;45(4):397–400. doi: 10.1042/bj0450397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor D. J. A technique for chronic catheterization of the amniotic and allantoic sacs of sheep foetuses. Res Vet Sci. 1970 Jan;11(1):93–95. [PubMed] [Google Scholar]
- Mellor D. J. Distribution of ions and electrical potential differences between mother and foetus at different gestational ages in goats and sheep. J Physiol. 1970 Mar;207(1):133–150. doi: 10.1113/jphysiol.1970.sp009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor D. J. Potential differences between mother and foetus at different gestational ages in the rat, rabbit and guinea-pig. J Physiol. 1969 Oct;204(2):395–405. doi: 10.1113/jphysiol.1969.sp008919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor D. J. Vascular anastomosis and fusion of foetal membranes in multiple pregnancy in the sheep. Res Vet Sci. 1969 Jul;10(4):361–367. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Slater J. S., Dunnett E. J. Determination of total amino acid concentrations in plasma. Proc Nutr Soc. 1970 May;29(1):104–105. doi: 10.1079/pns19700022. [DOI] [PubMed] [Google Scholar]


