Abstract
1. The resting membrane potential of Ascaris muscle fibres, which is normally relatively insensitive to ion changes in the medium, has been measured under a wide variety of conditions.
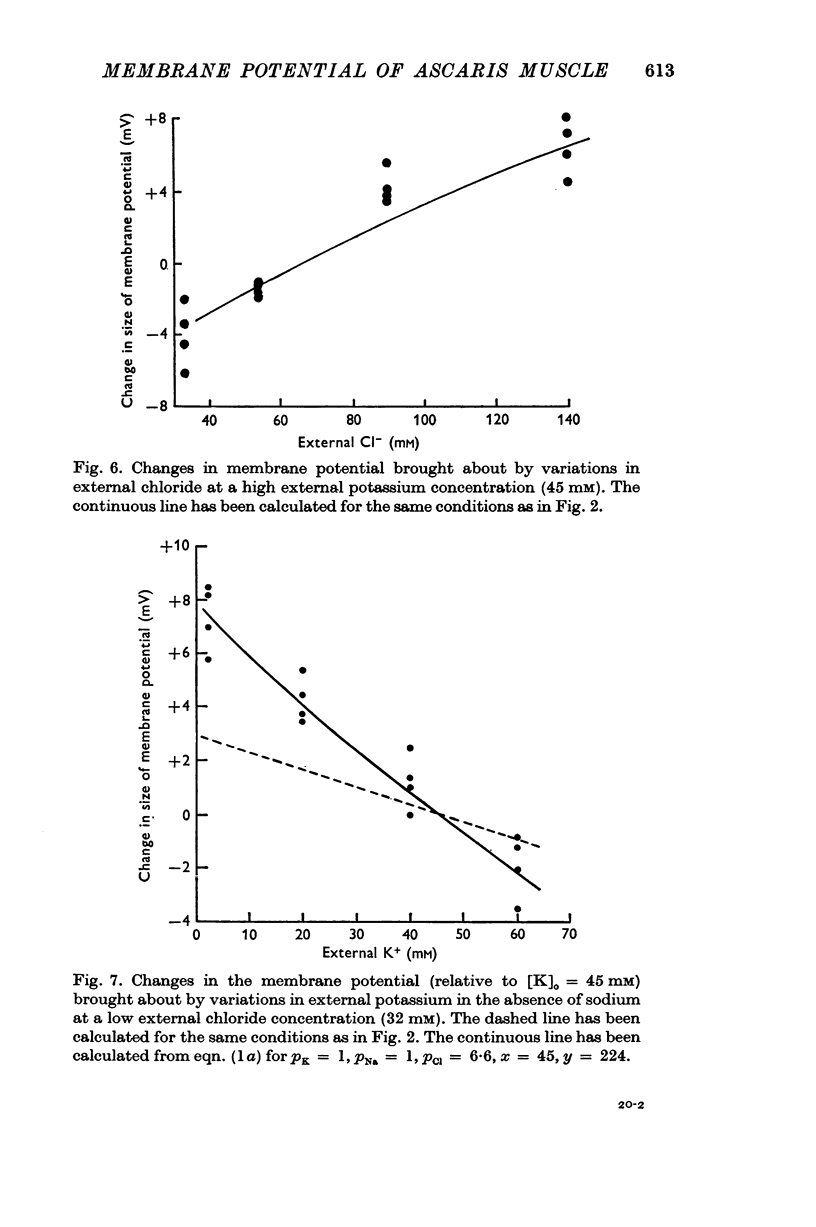
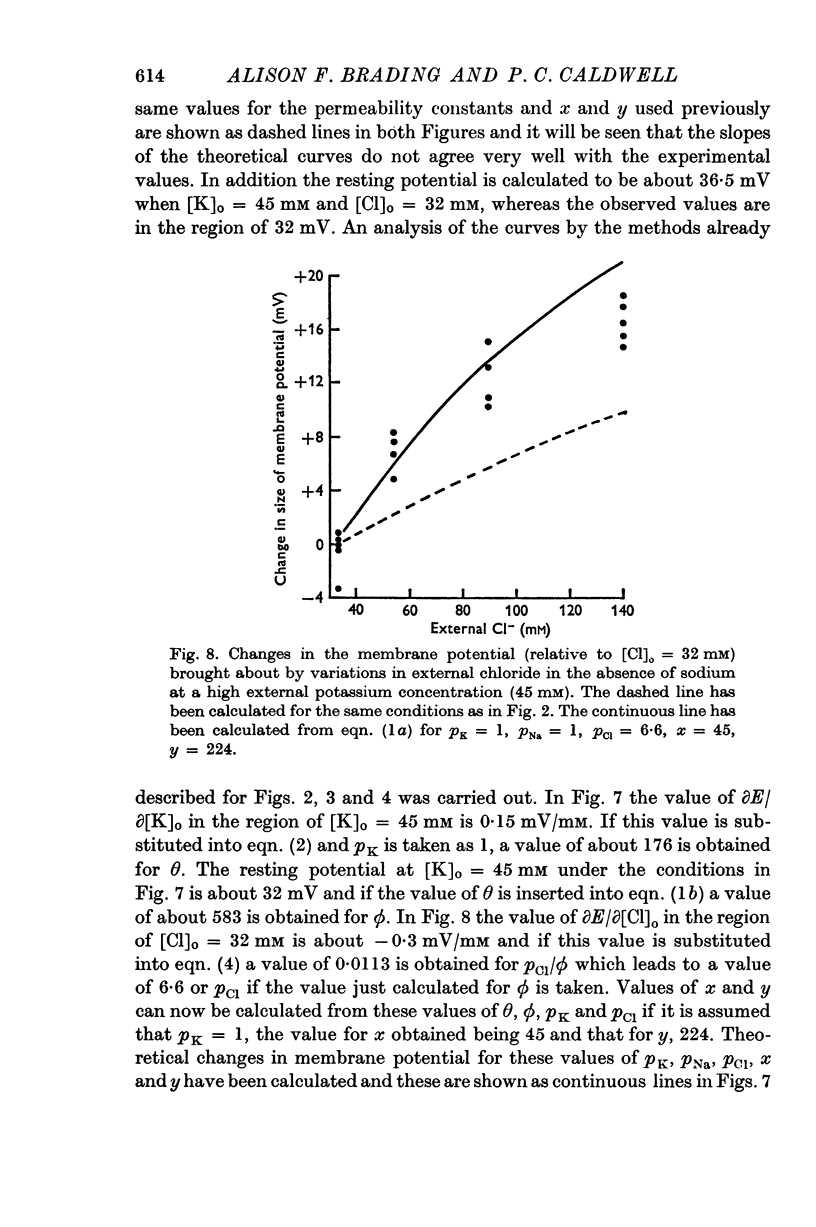
2. The results have been interpreted in terms of a form of the constant field equation containing additional terms for the contribution of ions and charged groups other than potassium, sodium and chloride.
3. Normally the contribution of the additional terms is large and tends to outweigh the contributions of potassium, sodium and chloride.
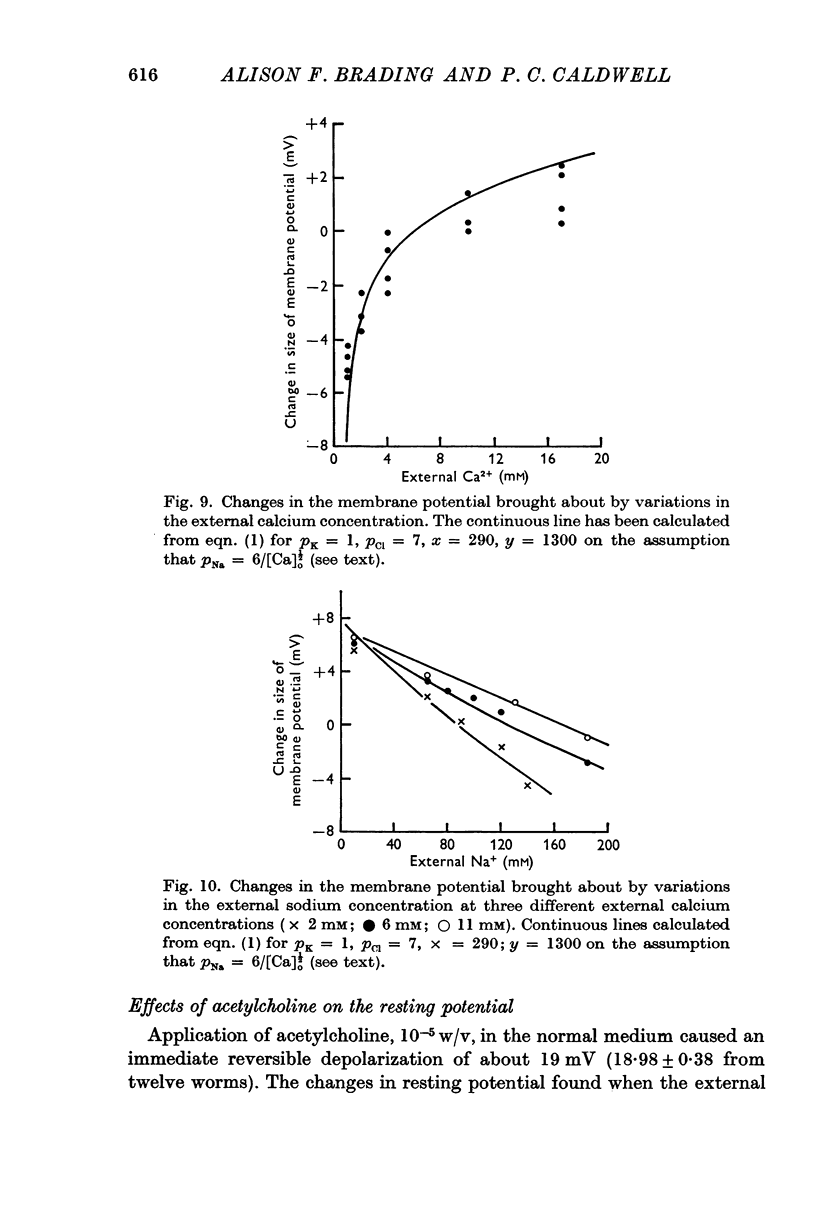
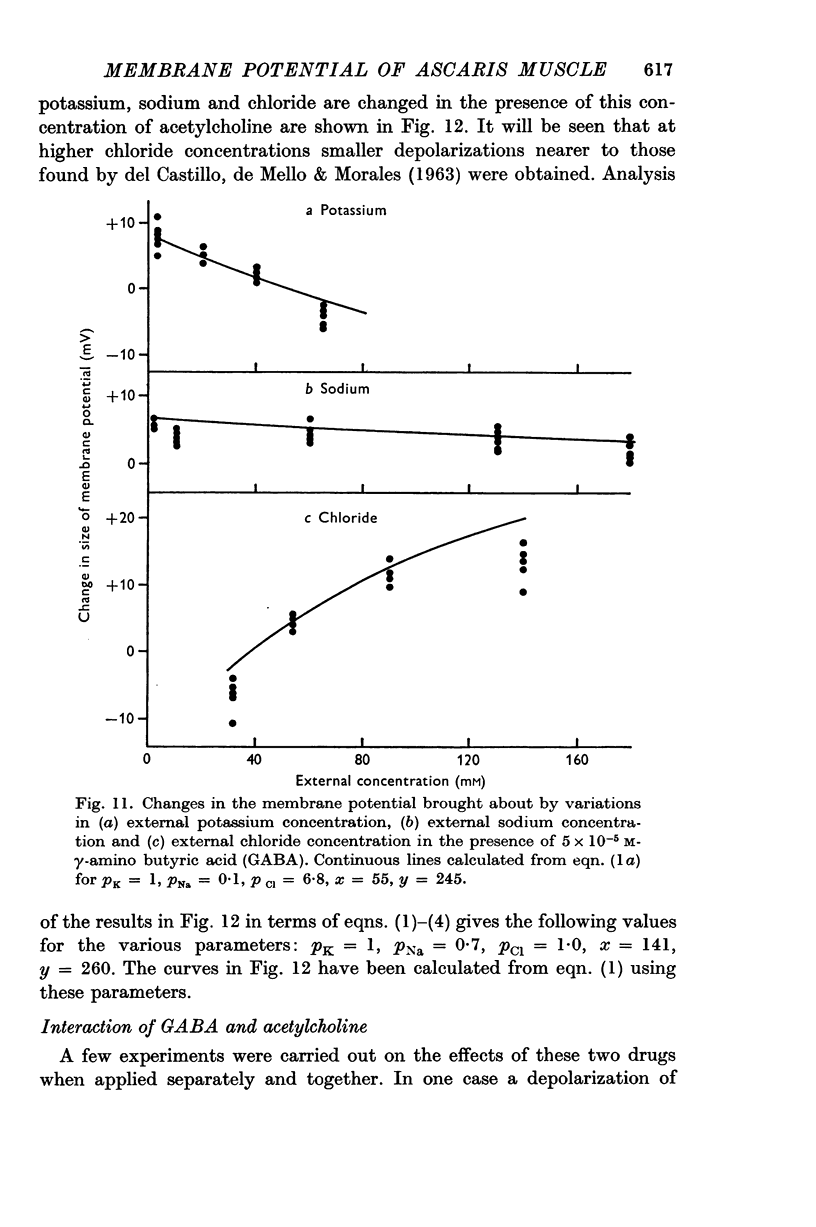
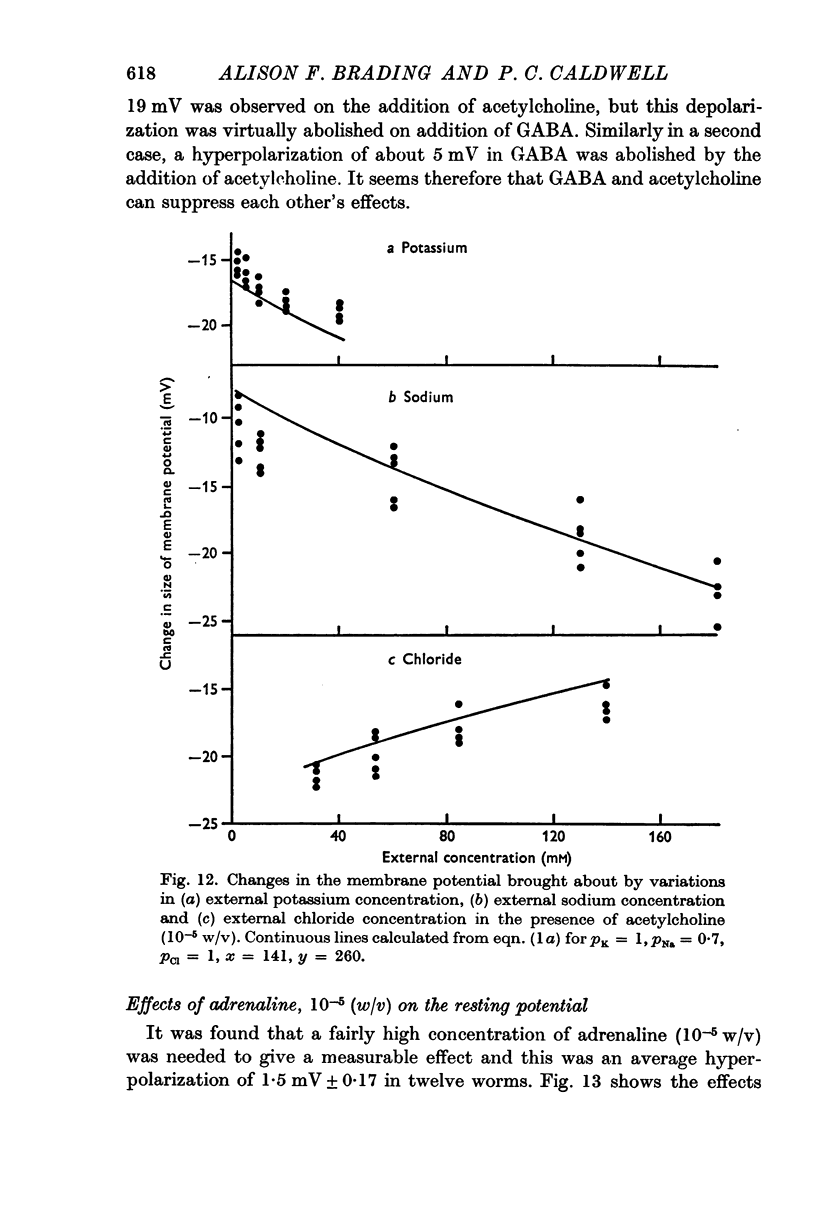
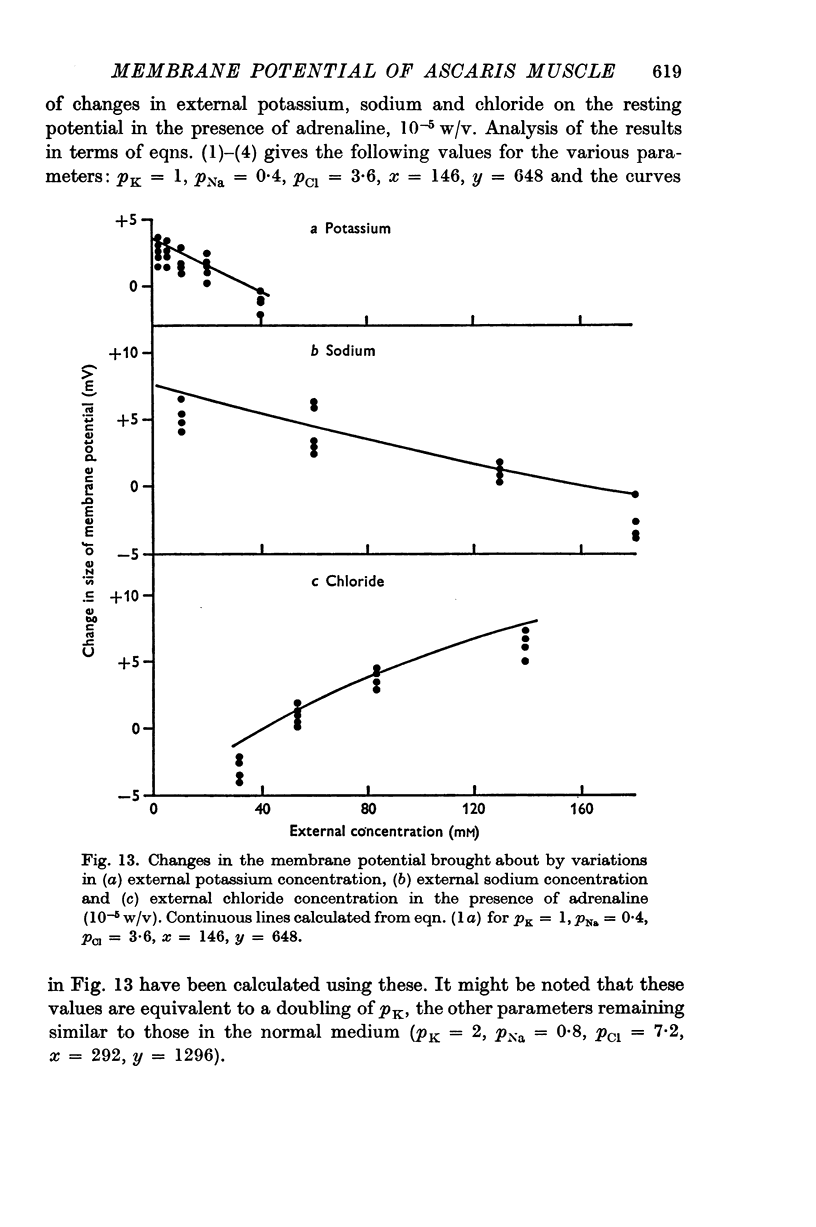
4. The contribution of the additional terms is considerably reduced in the absence of sodium and in the presence of γ-amino butyric acid and acetylcholine.
5. It is suggested that the additional terms may represent the contribution of an electrogenic active transport mechanism to the factors determining the membrane potential.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W. C., MORALES T. INFLUENCE OF SOME IONS ON THE MEMBRANE POTENTIAL OF ASCARIS MUSCLE. J Gen Physiol. 1964 Sep;48:129–140. doi: 10.1085/jgp.48.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W. C., MORALES T. MECHANISM OF THE PARALYSING ACTION OF PIPERAZINE ON ASCARIS MUSCLE. Br J Pharmacol Chemother. 1964 Jun;22:463–477. doi: 10.1111/j.1476-5381.1964.tb01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J., De Mello W. C., Morales T. Inhibitory action of gamma-aminobutyric acid (GABA) on Ascaris muscle. Experientia. 1964 Mar 15;20(3):141–143. doi: 10.1007/BF02150701. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D. Analysis of membrane permeability coefficient ratios and internal ion concentrations from a constant field equation. J Theor Biol. 1968 Apr;19(1):67–78. doi: 10.1016/0022-5193(68)90005-2. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton R. B. An investigation of the electrogenic sodium pump in snail neurones, using the constant-field theory. J Exp Biol. 1969 Aug;51(1):181–201. doi: 10.1242/jeb.51.1.181. [DOI] [PubMed] [Google Scholar]


