Abstract
1. An analysis has been made of the distribution and rise time of the responses induced by electrophoretic application of ACh on junctional and extrajunctional receptors of the neuromuscular junction of the frog.
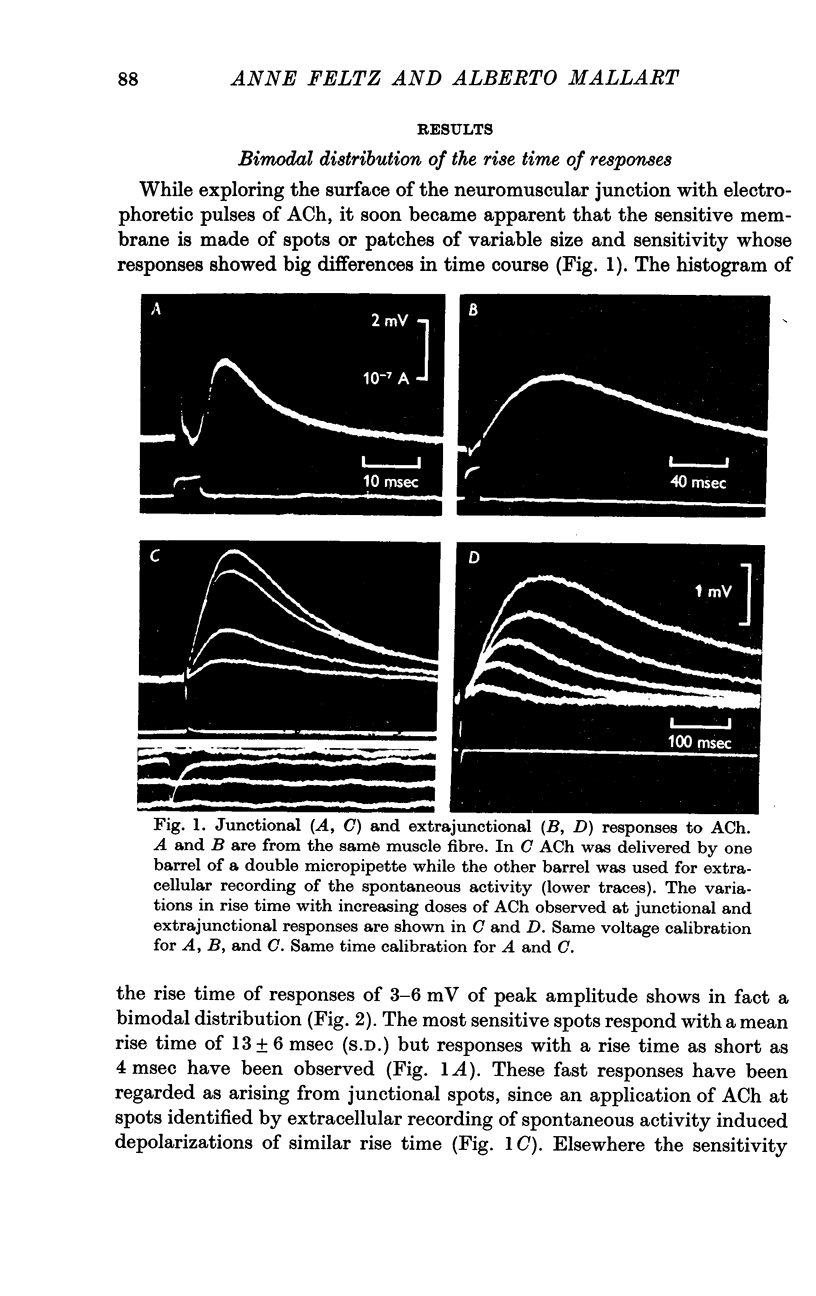
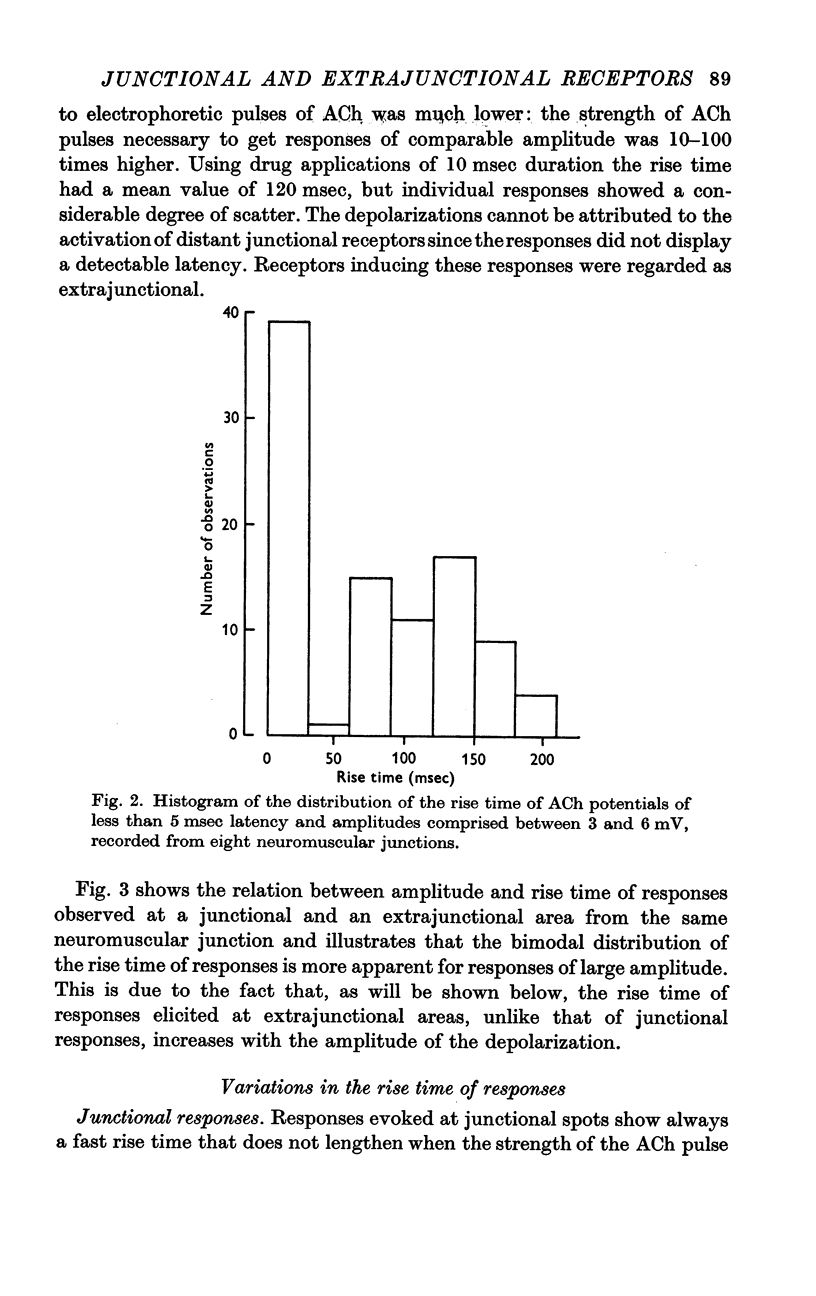
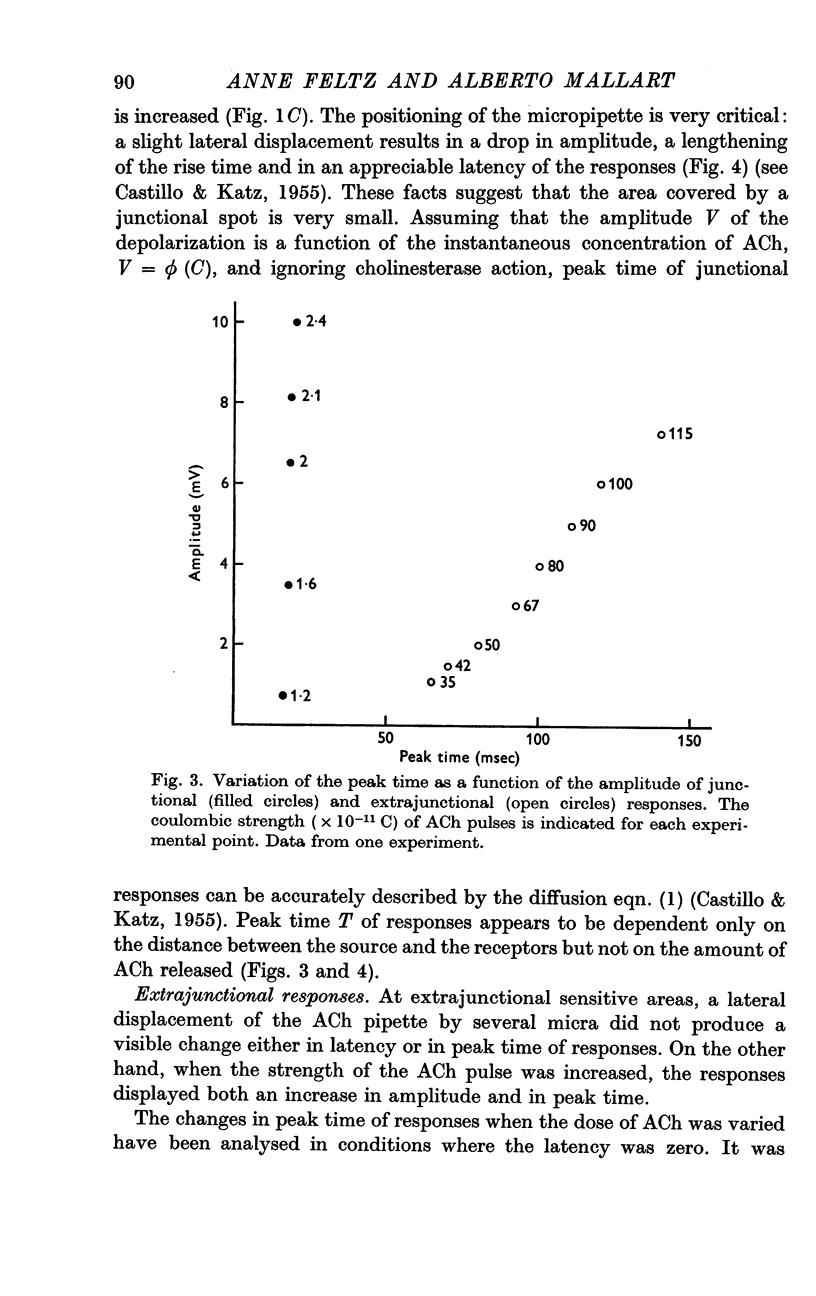
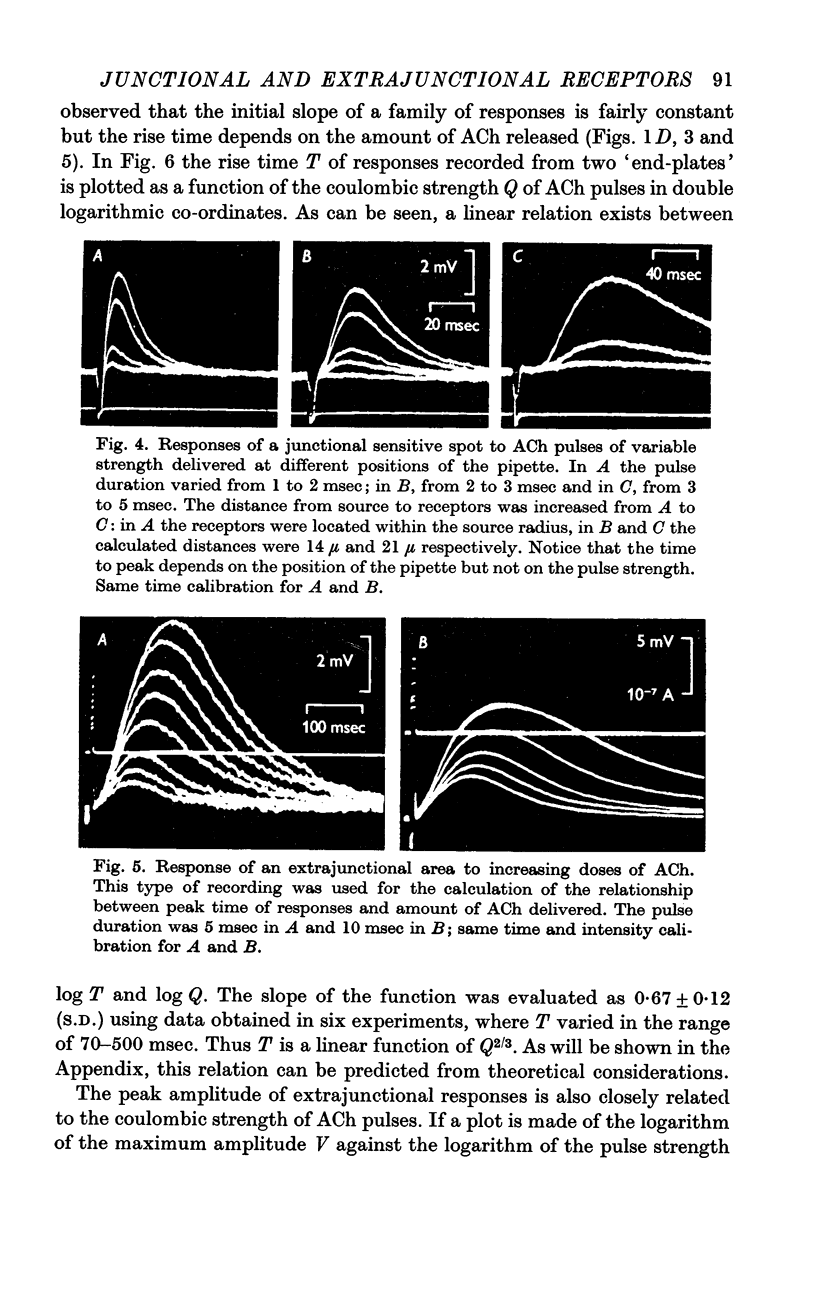
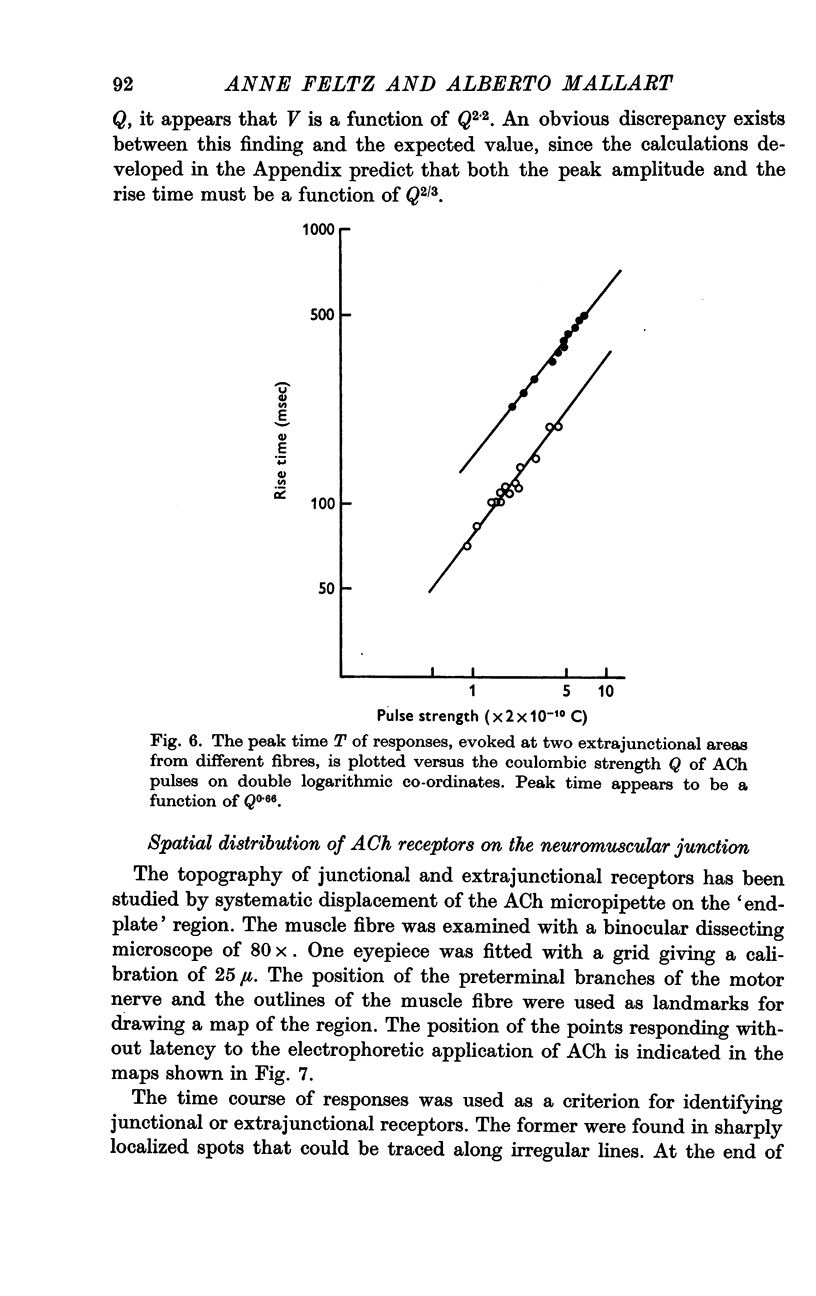
2. The junctional and the extrajunctional responses appear to constitute two independent groups with no overlapping characteristics: (a) the responses evoked from junctional receptors show always a high `sensitivity' (30-100 mV/nC) and a rise time usually shorter than 10 msec that does not lengthen as the ACh dose is increased; (b) the responses evoked from extrajunctional area show a lower sensitivity (0·5-10 mV/nC) and a slow rise time that is a linear function of the dose Q⅔ of ACh.
3. The lengthening of the rise time of extrajunctional response with increasing doses of ACh is interpreted by supposing that larger doses saturate larger areas of a membrane of low sensitivity uniformly covered with receptors. On the contrary, at junctional spots the does of ACh would be insufficient to saturate the receptors and rise time will be independent of dose.
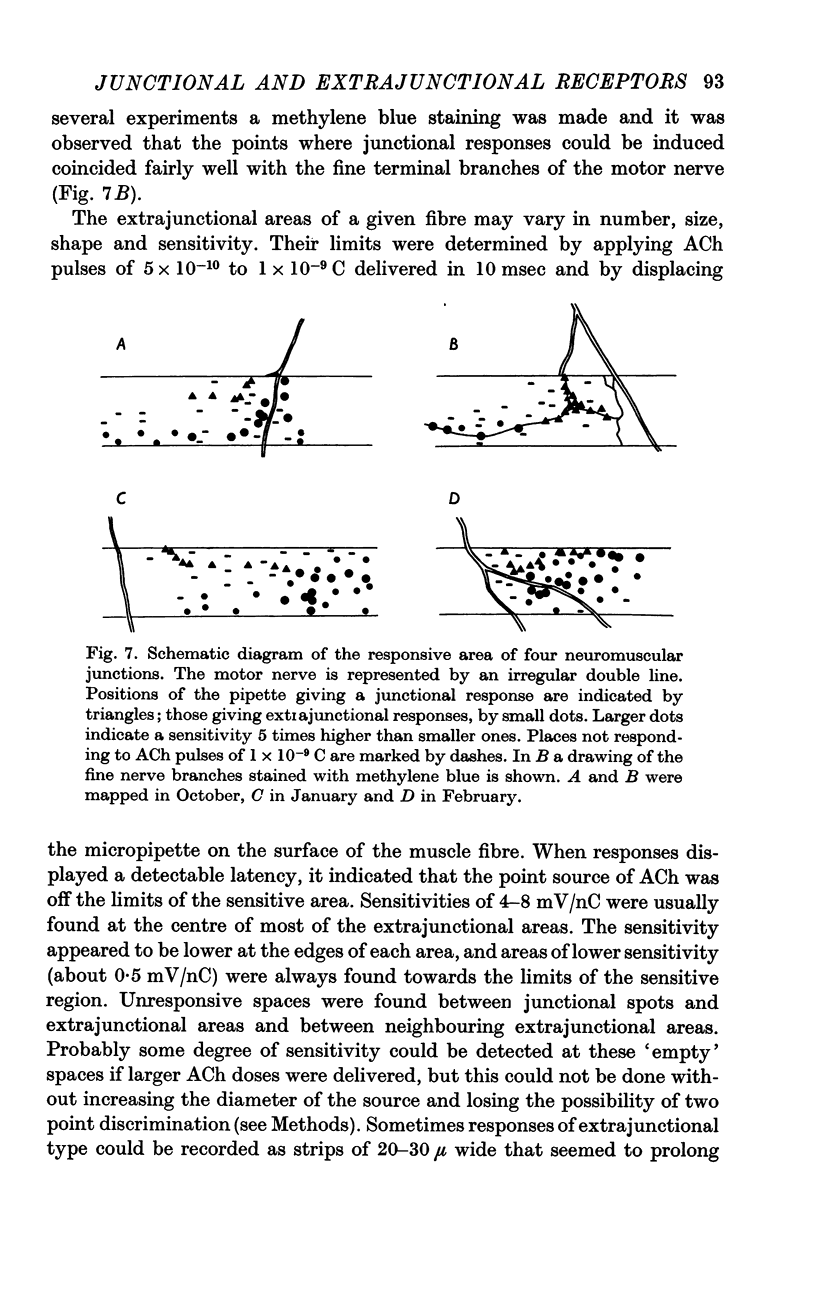
4. Relatively unresponsive zones may exist between junctional spots and extrajunctional areas and between neighbouring extrajunctional areas.
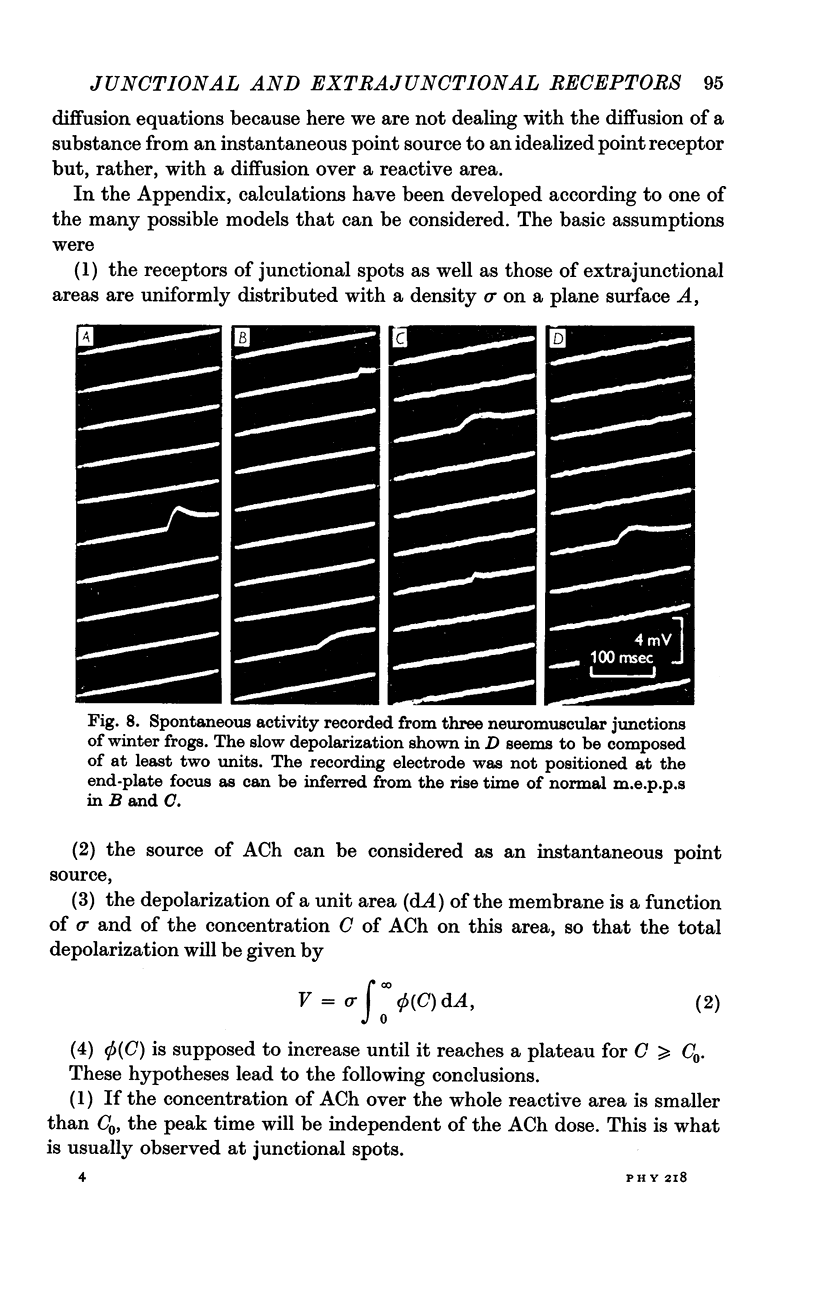
5. Extrajunctional areas are subjected to significant seasonal variations; in summer they are practically absent, in autumn they appear as elongated areas of about 20 μ wide and 50 μ long, and late in winter they fuse together and all the `end-plate' zone becomes sensitive to ACh.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Braun M., Schmidt R. F., Zimmermann M. Facilitation at the frog neuromuscular junction during and after repetitive stimulation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):41–55. doi: 10.1007/BF00362453. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. FURTHER OBSERVATIONS ON THE DISTRIBUTION OF ACTYLCHOLINE-REACTIVE SITES IN SKELETAL MUSCLE. J Physiol. 1964 Mar;170:379–388. doi: 10.1113/jphysiol.1964.sp007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Maeno T. Analysis of mobilization and demobilization processes in neuromuscular transmission in the frog. J Neurophysiol. 1969 Sep;32(5):793–800. doi: 10.1152/jn.1969.32.5.793. [DOI] [PubMed] [Google Scholar]
- Mallart A., Feltz A. Mise en jeu de perméabilités ioniques distinctes au niveau des récepteurs synaptiques et extrasynaptiques des fibres musculaires striées. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jun 2;268(22):2724–2726. [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- Vrbová G. Induction of an extrajunctional chemosensitive area in intact innervated muscle fibres. J Physiol. 1967 Jul;191(1):20P–21P. [PubMed] [Google Scholar]


