Abstract
1. The isolated renal papillae of a rat were centrifuged in a two tube assembly which allowed fluid from the tissue to separate into the lower tube.
2. The papillae were centrifuged for 15 min at 300 g and 1500 g consecutively.
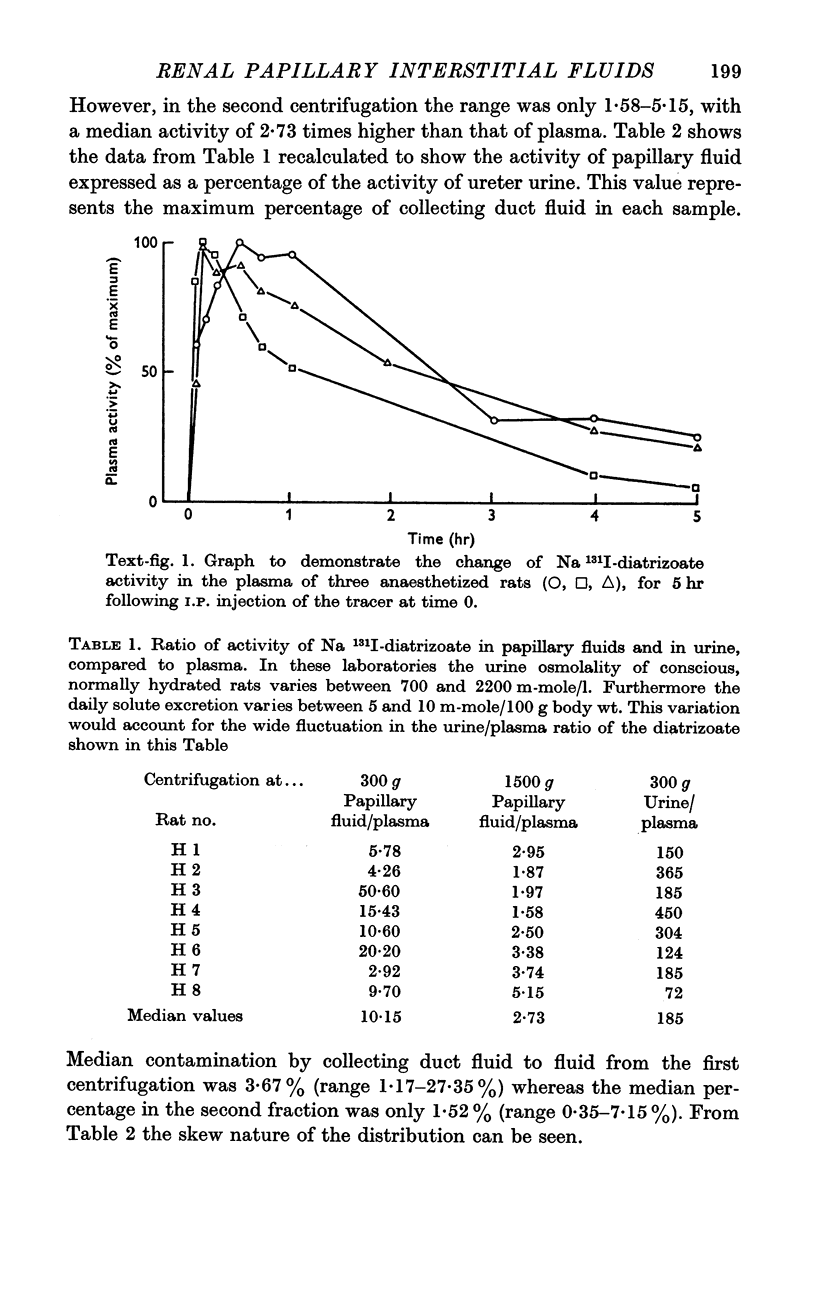
3. After intraperitoneal injection of Na 131I-diatrizoate, the activities of urine, and fluid samples obtained from the papilla, were compared. It was found at 1500 g that the median value for papillary fluid activity was 1·52% of the activity of urine. This is evidence that the papillary fluid was virtually free from any contamination from the terminal collecting ducts.
4. It is considered that the fluid sample obtained from the papillae by centrifugation at 1500 g is a representative and reproducible sample of interstitial fluid.
5. The method was used to demonstrate changes in solute concentrations in the renal papillary fluid, following dehydration of rats.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C., Green R., Thomas S. Influence of lysine-vasopressin dosage on the time course of changes in renal tissue and urinary composition in the conscious rat. J Physiol. 1971 Mar;213(2):291–309. doi: 10.1113/jphysiol.1971.sp009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., GORESKY C. A., NOLAN M. F. RENAL TRANSIT TIMES AND DISTRIBUTION VOLUMES OF T-1824, CREATININE, AND WATER. Am J Physiol. 1965 Aug;209:243–252. doi: 10.1152/ajplegacy.1965.209.2.243. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MYLLE M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959 Apr;196(4):927–936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W. OSMOTIC CONCENTRATION AND DILUTION OF THE URINE. Am J Med. 1964 May;36:670–685. doi: 10.1016/0002-9343(64)90179-2. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Bennett C. M., Berliner R. W. Countercurrent multiplication by the thin loops of Henle. Am J Physiol. 1967 Feb;212(2):357–366. doi: 10.1152/ajplegacy.1967.212.2.357. [DOI] [PubMed] [Google Scholar]
- Kettyle W. M., Horster M., Thurau K., Valtin H. Cryoscopic determination of renal tissue osmolality by an ultramicro method. Pflugers Arch. 1970;321(1):83–89. doi: 10.1007/BF00594124. [DOI] [PubMed] [Google Scholar]
- LEVITIN H., GOODMAN A., PIGEON G., EPSTEIN F. H. Composition of the renal medulla during water diuresis. J Clin Invest. 1962 May;41:1145–1151. doi: 10.1172/JCI104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Morgan T., Williams P. G. Sodium and urea concentrations in renal papillary fluid of rats, with dehydration and vasopressin (Pitressin) administration. J Physiol. 1971 May;215(1):41P–42P. [PubMed] [Google Scholar]
- Marsh D. J. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol. 1970 Mar;218(3):824–831. doi: 10.1152/ajplegacy.1970.218.3.824. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- SAIKIA T. C. THE ACUTE EFFECT OF VASOPRESSIN UPON THE COMPOSITION OF THE RAT RENAL CORTEX AND MEDULLA. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:158–168. doi: 10.1113/expphysiol.1965.sp001778. [DOI] [PubMed] [Google Scholar]
- Valtin H. Sequestration of urea and nonurea solutes in renal tissues of rats with hereditary hypothalamic diabetes insipidus: effect of vasopressin and dehydration on the countercurrent mechanism. J Clin Invest. 1966 Mar;45(3):337–345. doi: 10.1172/JCI105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRZ H., HARGITAY B., KUHN W. Lokalisation des Konzentrierungsprozesses in der Niere durch direkte Kryoskopie. Helv Physiol Pharmacol Acta. 1951 Jun;9(2):196–207. [PubMed] [Google Scholar]




